A novel strategy for SARS-CoV-2 mass screening with quantitative antigen testing of saliva: a diagnostic accuracy study
- PMID: 34031649
- PMCID: PMC8133768
- DOI: 10.1016/S2666-5247(21)00092-6
A novel strategy for SARS-CoV-2 mass screening with quantitative antigen testing of saliva: a diagnostic accuracy study
Abstract
Background: Quantitative RT-PCR (RT-qPCR) of nasopharyngeal swab (NPS) samples for SARS-CoV-2 detection requires medical personnel and is time consuming, and thus is poorly suited to mass screening. In June, 2020, a chemiluminescent enzyme immunoassay (CLEIA; Lumipulse G SARS-CoV-2 Ag kit, Fujirebio, Tokyo, Japan) was developed that can detect SARS-CoV-2 nucleoproteins in NPS or saliva samples within 35 min. In this study, we assessed the utility of CLEIA in mass SARS-CoV-2 screening.
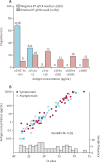
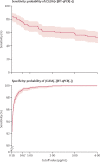
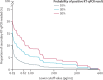
Methods: We did a diagnostic accuracy study to develop a mass-screening strategy for salivary detection of SARS-CoV-2 by CLEIA, enrolling hospitalised patients with clinically confirmed COVID-19, close contacts identified at community health centres, and asymptomatic international arrivals at two airports, all based in Japan. All test participants were enrolled consecutively. We assessed the diagnostic accuracy of CLEIA compared with RT-qPCR, estimated according to concordance (Kendall's coefficient of concordance, W), and sensitivity (probability of CLEIA positivity given RT-qPCR positivity) and specificity (probability of CLEIA negativity given RT-qPCR negativity) for different antigen concentration cutoffs (0·19 pg/mL, 0·67 pg/mL, and 4·00 pg/mL; with samples considered positive if the antigen concentration was equal to or more than the cutoff and negative if it was less than the cutoff). We also assessed a two-step testing strategy post hoc with CLEIA as an initial test, using separate antigen cutoff values for test negativity and positivity from the predefined cutoff values. The proportion of intermediate results requiring secondary RT-qPCR was then quantified assuming prevalence values of RT-qPCR positivity in the overall tested population of 10%, 30%, and 50%.
Findings: Self-collected saliva was obtained from 2056 participants between June 12 and Aug 6, 2020. Results of CLEIA and RT-qPCR were concordant in 2020 (98·2%) samples (Kendall's W=0·99). Test sensitivity was 85·4% (76 of 89 positive samples; 90% credible interval [CrI] 78·0-90·3) at the cutoff of 0·19 pg/mL; 76·4% (68 of 89; 68·2-82·8) at the cutoff of 0·67 pg/mL; and 52·8% (47 of 89; 44·1-61·3) at the cutoff of 4·0 pg/mL. Test specificity was 91·3% (1796 of 1967 negative samples; 90% CrI 90·2-92·3) at the cutoff of 0·19 pg/mL, 99·2% (1952 of 1967; 98·8-99·5) at the cutoff of 0·67 pg/mL, and 100·0% (1967 of 1967; 99·8-100·0) at the cutoff of 4·00 pg/mL. Using a two-step testing strategy with a CLEIA negativity cutoff of 0·19 pg/mL (to maximise sensitivity) and a CLEIA positivity cutoff of 4·00 pg/mL (to maximise specificity), the proportions of indeterminate results (ie, samples requiring secondary RT-qPCR) would be approximately 11% assuming a prevalence of RT-qPCR positivity of 10%, 16% assuming a prevalence of RT-qPCR positivity of 30%, and 21% assuming a prevalence of RT-qPCR positivity of 50%.
Interpretation: CLEIA testing of self-collected saliva is simple and provides results quickly, and is thus suitable for mass testing. To improve accuracy, we propose a two-step screening strategy with an initial CLEIA test followed by confirmatory RT-qPCR for intermediate concentrations, varying positive and negative thresholds depending on local prevalence. Implementation of this strategy has expedited sample processing at Japanese airports since July, 2020, and might apply to other large-scale mass screening initiatives.
Funding: Ministry of Health, Labour and Welfare, Japan.
© 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Conflict of interest statement
IY reports a policy research grant from the Ministry of Health, Labour and Welfare, Japan, during the conduct of the study; and personal fees from Chugai Pharmaceutical, AstraZeneca, Japan Tobacco Pharmaceutical Division, and Nippon Shinyaku, outside the submitted work. PYS reports personal fees from AYUMI Pharmaceutical, Japan Pharmaceutical Manufacturers Association, Alexion Pharmaceuticals, and Kyowa Kirin, outside the submitted work. YU reports a policy research grant from the Ministry of Health, Labour and Welfare, Japan, during the conduct of the study. YY reports a policy research grant from the Ministry of Health, Labour and Welfare, Japan, during the conduct of the study. TT reports policy research grant from the Ministry of Health, Labour and Welfare, Japan, during the conduct of the study; personal fees from Merck Sharp & Dohme, Takeda Pharmaceutical, Pfizer Japan, and Bristol Myers Squibb, grants and personal fees from Kyowa Hakko Kirin, grants, personal fees, and non-financial support from Novartis Pharma, grants from Chugai Pharmaceutical, Sanofi, Astellas Pharma, Teijin Pharma, Fuji Pharma, Nippon Shinyaku, the Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research), and the Center of Innovation Program of the Japan Science and Technology Agency, and non-financial support from Janssen Pharmaceutical, outside the submitted work. All other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

