Production of Rainbow Colorants by Metabolically Engineered Escherichia coli
- PMID: 34032018
- PMCID: PMC8261500
- DOI: 10.1002/advs.202100743
Production of Rainbow Colorants by Metabolically Engineered Escherichia coli
Abstract
There has been much interest in producing natural colorants to replace synthetic colorants of health concerns. Escherichia coli has been employed to produce natural colorants including carotenoids, indigo, anthocyanins, and violacein. However, production of natural green and navy colorants has not been reported. Many natural products are hydrophobic, which are accumulated inside or on the cell membrane. This causes cell growth limitation and consequently reduces production of target chemicals. Here, integrated membrane engineering strategies are reported for the enhanced production of rainbow colorants-three carotenoids and four violacein derivatives-as representative hydrophobic natural products in E. coli. By integration of systems metabolic engineering, cell morphology engineering, inner- and outer-membrane vesicle formation, and fermentation optimization, production of rainbow colorants are significantly enhanced to 322 mg L-1 of astaxanthin (red), 343 mg L-1 of β-carotene (orange), 218 mg L-1 of zeaxanthin (yellow), 1.42 g L-1 of proviolacein (green), 0.844 g L-1 of prodeoxyviolacein (blue), 6.19 g L-1 of violacein (navy), and 11.26 g L-1 of deoxyviolacein (purple). The membrane engineering strategies reported here are generally applicable to microbial production of a broader range of hydrophobic natural products, contributing to food, cosmetic, chemical, and pharmaceutical industries.
Keywords: membrane engineering; metabolic engineering; natural products; rainbow colorants; vesicle.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
D.Y., S.Y.P., and S.Y.L. declare that the membrane engineering technologies described here are patent filed including, but not limited to KR 10‐2020‐0144521.
Figures
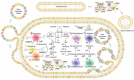
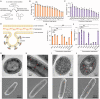
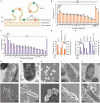

Similar articles
-
Metabolic engineering of the violacein biosynthetic pathway toward a low-cost, minimal-equipment lead biosensor.Biosens Bioelectron. 2022 Oct 15;214:114531. doi: 10.1016/j.bios.2022.114531. Epub 2022 Jul 6. Biosens Bioelectron. 2022. PMID: 35810697
-
Metabolic Engineering of Escherichia coli for Producing Astaxanthin as the Predominant Carotenoid.Mar Drugs. 2017 Sep 22;15(10):296. doi: 10.3390/md15100296. Mar Drugs. 2017. PMID: 28937591 Free PMC article.
-
Systems metabolic engineering of Escherichia coli for the high-level production of deoxyviolacein, a natural colorant.Bioresour Technol. 2025 Sep;431:132584. doi: 10.1016/j.biortech.2025.132584. Epub 2025 Apr 24. Bioresour Technol. 2025. PMID: 40286824
-
Metabolic Engineering of Escherichia coli for Natural Product Biosynthesis.Trends Biotechnol. 2020 Jul;38(7):745-765. doi: 10.1016/j.tibtech.2019.11.007. Epub 2020 Jan 7. Trends Biotechnol. 2020. PMID: 31924345 Review.
-
Recent advances in engineering microorganisms for the production of natural food colorants.Curr Opin Chem Biol. 2024 Aug;81:102477. doi: 10.1016/j.cbpa.2024.102477. Epub 2024 Jun 14. Curr Opin Chem Biol. 2024. PMID: 38878611 Review.
Cited by
-
Allergenic risk assessment of porcine myoglobin expressed by engineered Komagataella Phaffii.Fundam Res. 2024 Jan 27;4(5):1339-1348. doi: 10.1016/j.fmre.2023.11.017. eCollection 2024 Sep. Fundam Res. 2024. PMID: 39431137 Free PMC article.
-
Stability of a Mutualistic Escherichia coli Co-Culture During Violacein Production Depends on the Kind of Carbon Source.Eng Life Sci. 2024 Sep 8;24(10):e202400025. doi: 10.1002/elsc.202400025. eCollection 2024 Oct. Eng Life Sci. 2024. PMID: 39391271 Free PMC article.
-
Optimization of microbial cell factories for astaxanthin production: Biosynthesis and regulations, engineering strategies and fermentation optimization strategies.Synth Syst Biotechnol. 2022 Feb 18;7(2):689-704. doi: 10.1016/j.synbio.2022.01.002. eCollection 2022 Jun. Synth Syst Biotechnol. 2022. PMID: 35261927 Free PMC article. Review.
-
Recent Advances in Synthetic, Industrial and Biological Applications of Violacein and Its Heterologous Production.J Microbiol Biotechnol. 2021 Nov 28;31(11):1465-1480. doi: 10.4014/jmb.2107.07045. J Microbiol Biotechnol. 2021. PMID: 34584039 Free PMC article. Review.
-
Biotechnological advances for improving natural pigment production: a state-of-the-art review.Bioresour Bioprocess. 2022 Jan 28;9(1):8. doi: 10.1186/s40643-022-00497-4. Bioresour Bioprocess. 2022. PMID: 38647847 Free PMC article. Review.
References
-
- Batada A., Jacobson M. F., Clin. Pediatr. 2016, 55, 1113. - PubMed
-
- Kant R., Nat. Sci. 2012, 4, 22.
-
- a) Lee S. Y., Kim H. U., Chae T. U., Cho J. S., Kim J. W., Shin J. H., Kim D. I., Ko Y. S., Jang W. D., Jang Y. S., Nat. Catal. 2019, 2, 18;
- b) Nielsen J., Keasling J. D., Cell 2016, 164, 1185; - PubMed
- c) Yang D., Park S. Y., Park Y. S., Eun H., Lee S. Y., Trends Biotechnol. 2020, 38, 745; - PubMed
- d) Cravens A., Payne J., Smolke C. D., Nat. Commun. 2019, 10, 2142. - PMC - PubMed
-
- Hug J. J., Krug D., Muller R., Nat. Rev. Chem. 2020, 4, 172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
