Efficacy and Safety of Yukgunja-Tang for Patients with Cancer-related Anorexia: A Randomized, Controlled Trial, Pilot Study
- PMID: 34032151
- PMCID: PMC8371029
- DOI: 10.1177/15347354211019107
Efficacy and Safety of Yukgunja-Tang for Patients with Cancer-related Anorexia: A Randomized, Controlled Trial, Pilot Study
Abstract
Objective: The purpose of this study is both to estimate the efficacy and the safety of Yukgunja-tang (YGJT) and to establish evidence for the use of herbal medicines in the management of patients with cancer-related anorexia.
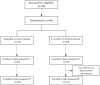
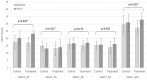
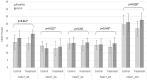
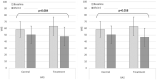
Methods: We enrolled 40 patients with cancer-related anorexia. The enrolled participants were randomly allocated to 2 groups: the control group (n = 20), which received nutrition counseling, and the treatment group (n = 20), which received nutrition counseling and was administered YGJT at twice a day for 4 weeks (a total of 56 times @ 3.0 g each time). The primary outcome of this study was the score on the anorexia/cachexia subscale (ACS) of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT). The secondary outcomes were the FAACT score with the ACS score excluded, the score on the Visual Analog Scale (VAS) for appetite, and the results on laboratory tests regarding appetite, such as leptin, tumor necrosis factors (TNF-α), interleukin-6 (IL-6), and ghrelin. All variables related to the safety assessment, such as vital signs, electrocardiography results, laboratory test results (complete blood cell count, chemistry, urine test), and adverse events, were documented on the case report form (CRF) at every visit.
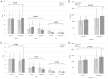
Result: The difference in the primary outcome, that is, the score on the anorexia/cachexia subscale (ACS) of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT), between the control and the treatment groups was statistically significant (P = .023) as was the difference in the FAACT scores with the ACS score excluded, a secondary outcome, between the 2 groups; however, no statistically significant differences were noted in the scores on the VAS or the levels of leptin, TNF-α, IL-6, and ghrelin. In addition, no significant differences in the numbers and the types of adverse events or in the results on the laboratory tests between the control and the treatment groups were recorded.
Conclusion: These results obtained in this research confirmed the efficacy and the safety of using YGJT as a herb-medicine treatment option for patients with cancer-related anorexia.
Keywords: Liu-Jun-Zi-Tang; Rikkunshito; Yukgunja-Tang; anorexia; cancer; clinical trials; malnutrition.
Conflict of interest statement
Figures
References
-
- Mantovani G, Macciò A, Massa E, Madeddu C.Managing cancer-related anorexia/cachexia. Drugs. 2001;61:499-514. - PubMed
-
- Mattox TW.Cancer cachexia: cause, diagnosis, and treatment. Nutr Clin Pract. 2017;32:599-606. - PubMed
-
- Brown JK.A systematic review of the evidence on symptom management of cancer-related anorexia and cachexia. Oncol Nurs Forum. 2002;29:517-532. - PubMed
-
- Wie GA, Cho YA, Kim SY, Kim SM, Bae JM, Joung H.Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition. 2010;26:263-268. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

