Postnatal prolongation of mammalian nephrogenesis by excess fetal GDNF
- PMID: 34032268
- PMCID: PMC8180252
- DOI: 10.1242/dev.197475
Postnatal prolongation of mammalian nephrogenesis by excess fetal GDNF
Abstract
Nephron endowment, defined during the fetal period, dictates renal and related cardiovascular health throughout life. We show here that, despite its negative effects on kidney growth, genetic increase of GDNF prolongs the nephrogenic program beyond its normal cessation. Multi-stage mechanistic analysis revealed that excess GDNF maintains nephron progenitors and nephrogenesis through increased expression of its secreted targets and augmented WNT signaling, leading to a two-part effect on nephron progenitor maintenance. Abnormally high GDNF in embryonic kidneys upregulates its known targets but also Wnt9b and Axin2, with concomitant deceleration of nephron progenitor proliferation. Decline of GDNF levels in postnatal kidneys normalizes the ureteric bud and creates a permissive environment for continuation of the nephrogenic program, as demonstrated by morphologically and molecularly normal postnatal nephron progenitor self-renewal and differentiation. These results establish that excess GDNF has a bi-phasic effect on nephron progenitors in mice, which can faithfully respond to GDNF dosage manipulation during the fetal and postnatal period. Our results suggest that sensing the signaling activity level is an important mechanism through which GDNF and other molecules contribute to nephron progenitor lifespan specification.
Keywords: Differentiation; Kidney; Mouse; Nephrogenesis; Nephron progenitors.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
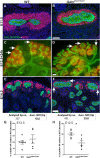

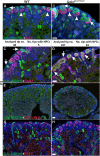



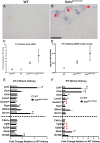


References
-
- Barak, H., Huh, S.-H., Chen, S., Jeanpierre, C., Martinovic, J., Parisot, M., Bole-Feysot, C., Nitschké, P., Salomon, R., Antignac, C.et al. (2012). FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev. Cell 22, 1191-1207. 10.1016/j.devcel.2012.04.018 - DOI - PMC - PubMed
-
- Boyle, S., Misfeldt, A., Chandler, K. J., Deal, K. K., Southard-Smith, E. M., Mortlock, D. P., Baldwin, H. S. and de Caestecker, M. (2008). Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 313, 234-245. 10.1016/j.ydbio.2007.10.014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

