VLX1570 induces apoptosis through the generation of ROS and induction of ER stress on leukemia cell lines
- PMID: 34032336
- PMCID: PMC8353915
- DOI: 10.1111/cas.14982
VLX1570 induces apoptosis through the generation of ROS and induction of ER stress on leukemia cell lines
Abstract
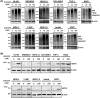
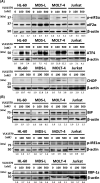
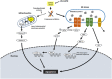
A novel proteasome deubiquitinase inhibitor, VLX1570, has been highlighted as a promising therapeutic agent mainly for lymphoid neoplasms and solid tumors. We examined in vitro effects of VLX1570 on eight myeloid and three lymphoid leukemia cell lines. From cell culture studies, 10 out of 11 cell lines except K562 were found to be susceptible to VLX1570 treatment and it inhibited cell growth mainly by apoptosis. Next, to identify the signaling pathways associated with apoptosis, we performed gene expression profiling using HL-60 with or without 50 nmol/L of VLX1570 for 3 hours and demonstrated that VLX1570 induced the genetic pathway involved in "heat shock transcription factor 1 (HSF1) activation", "HSF1 dependent transactivation", and "Regulation of HSF1 mediated heat shock response". VLX1570 increased the amount of high molecular weight polyubiquitinated proteins and the expression of HSP70 as the result of the suppression of ubiquitin proteasome system, the expression of heme oxygenase-1, and the amount of phosphorylation in JNK and p38 associated with the generation of reactive oxygen species (ROS) induced apoptosis and the amount of phosphorylation in eIF2α, inducing the expression of ATF4 and endoplasmic reticulum (ER) stress dependent apoptosis protein, CHOP, and the amount of phosphorylation slightly in IRE1α, leading to increased expression of XBP-1s in leukemia cell lines. In the present study, we demonstrate that VLX1570 induces apoptosis and exerts a potential anti-leukemic effect through the generation of ROS and induction of ER stress in leukemia cell lines.
Keywords: VLX1570; acute myeloid leukemia; endoplasmic reticulum stress; proteasome deubiquitinase; reactive oxygen species.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



Similar articles
-
Induction of ER Stress in Acute Lymphoblastic Leukemia Cells by the Deubiquitinase Inhibitor VLX1570.Int J Mol Sci. 2020 Jul 4;21(13):4757. doi: 10.3390/ijms21134757. Int J Mol Sci. 2020. PMID: 32635430 Free PMC article.
-
Sensitivity of Acute Myelocytic Leukemia Cells to the Dienone Compound VLX1570 Is Associated with Inhibition of the Ubiquitin-Proteasome System.Biomolecules. 2021 Sep 10;11(9):1339. doi: 10.3390/biom11091339. Biomolecules. 2021. PMID: 34572552 Free PMC article.
-
Acute lymphoblastic leukemia cells are sensitive to disturbances in protein homeostasis induced by proteasome deubiquitinase inhibition.Oncotarget. 2017 Mar 28;8(13):21115-21127. doi: 10.18632/oncotarget.15501. Oncotarget. 2017. PMID: 28423502 Free PMC article.
-
The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells.Sci Rep. 2016 Jun 6;6:26979. doi: 10.1038/srep26979. Sci Rep. 2016. PMID: 27264969 Free PMC article.
-
Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia.J Leukoc Biol. 2013 Sep;94(3):423-9. doi: 10.1189/jlb.0113006. Epub 2013 May 28. J Leukoc Biol. 2013. PMID: 23715741 Free PMC article. Review.
Cited by
-
Is It Still Possible to Think about HSP70 as a Therapeutic Target in Onco-Hematological Diseases?Biomolecules. 2023 Mar 28;13(4):604. doi: 10.3390/biom13040604. Biomolecules. 2023. PMID: 37189352 Free PMC article. Review.
-
MG132-mediated Suppression of the Ubiquitin-proteasome Pathway Enhances the Sensitivity of Endometrial Cancer Cells to Cisplatin.Anticancer Agents Med Chem. 2025;25(4):281-291. doi: 10.2174/0118715206343550240919055701. Anticancer Agents Med Chem. 2025. PMID: 39354755
-
Targeting USP14/UCHL5: A Breakthrough Approach to Overcoming Treatment-Resistant FLT3-ITD-Positive AML.Int J Mol Sci. 2024 Sep 26;25(19):10372. doi: 10.3390/ijms251910372. Int J Mol Sci. 2024. PMID: 39408703 Free PMC article.
-
The emerging role of deubiquitylating enzymes as therapeutic targets in cancer metabolism.Cancer Cell Int. 2022 Mar 20;22(1):130. doi: 10.1186/s12935-022-02524-y. Cancer Cell Int. 2022. PMID: 35307036 Free PMC article. Review.
-
Reduced Proteolipid Protein 2 promotes endoplasmic reticulum stress-related apoptosis and increases drug sensitivity in acute myeloid leukemia.Mol Biol Rep. 2023 Dec 12;51(1):10. doi: 10.1007/s11033-023-08994-1. Mol Biol Rep. 2023. PMID: 38085372
References
-
- Arber DA, Orazi A, Hasserjian RP, et al. In: Swerdlow SH, Campo E, Harris NL, eds. Introduction and overview of the classification of myeloid neoplasms: WHO classification of tumours of Haematopoietic and Lymphoid Tissues, Revised, 4th edn. IARC: Lyon; 2017:16‐27.
-
- Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127:62‐70. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

