Protein Micropatterning in 2.5D: An Approach to Investigate Cellular Responses in Multi-Cue Environments
- PMID: 34032413
- PMCID: PMC8193632
- DOI: 10.1021/acsami.1c01984
Protein Micropatterning in 2.5D: An Approach to Investigate Cellular Responses in Multi-Cue Environments
Erratum in
-
Correction to "Protein Micropatterning in 2.5D: An Approach to Investigate Cellular Responses in Multi-Cue Environments".ACS Appl Mater Interfaces. 2022 Apr 6;14(13):15859. doi: 10.1021/acsami.2c04641. Epub 2022 Mar 24. ACS Appl Mater Interfaces. 2022. PMID: 35324140 Free PMC article. No abstract available.
Abstract
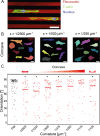
The extracellular microenvironment is an important regulator of cell functions. Numerous structural cues present in the cellular microenvironment, such as ligand distribution and substrate topography, have been shown to influence cell behavior. However, the roles of these cues are often studied individually using simplified, single-cue platforms that lack the complexity of the three-dimensional, multi-cue environment cells encounter in vivo. Developing ways to bridge this gap, while still allowing mechanistic investigation into the cellular response, represents a critical step to advance the field. Here, we present a new approach to address this need by combining optics-based protein patterning and lithography-based substrate microfabrication, which enables high-throughput investigation of complex cellular environments. Using a contactless and maskless UV-projection system, we created patterns of extracellular proteins (resembling contact-guidance cues) on a two-and-a-half-dimensional (2.5D) cell culture chip containing a library of well-defined microstructures (resembling topographical cues). As a first step, we optimized experimental parameters of the patterning protocol for the patterning of protein matrixes on planar and non-planar (2.5D cell culture chip) substrates and tested the technique with adherent cells (human bone marrow stromal cells). Next, we fine-tuned protein incubation conditions for two different vascular-derived human cell types (myofibroblasts and umbilical vein endothelial cells) and quantified the orientation response of these cells on the 2.5D, physiologically relevant multi-cue environments. On concave, patterned structures (curvatures between κ = 1/2500 and κ = 1/125 μm-1), both cell types predominantly oriented in the direction of the contact-guidance pattern. In contrast, for human myofibroblasts on micropatterned convex substrates with higher curvatures (κ ≥ 1/1000 μm-1), the majority of cells aligned along the longitudinal direction of the 2.5D features, indicating that these cells followed the structural cues from the substrate curvature instead. These findings exemplify the potential of this approach for systematic investigation of cellular responses to multiple microenvironmental cues.
Keywords: 2.5D substrate; cellular orientation; contact guidance; curvature; extracellular matrix; micropatterning; topography.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

