Bio-Inspired Amphoteric Polymer for Triggered-Release Drug Delivery on Breast Cancer Cells Based on Metal Coordination
- PMID: 34032419
- PMCID: PMC8381753
- DOI: 10.1021/acsami.1c03191
Bio-Inspired Amphoteric Polymer for Triggered-Release Drug Delivery on Breast Cancer Cells Based on Metal Coordination
Abstract
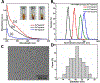
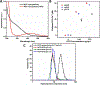
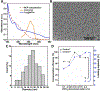
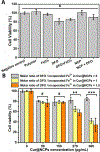
Nanoscale coordination polymers are promising vehicles for anticancer drug delivery because their surface composition and particle size can be tuned to exploit the enhanced permeability and retention effect, and their reversible interaction with metal cations enables triggered drug release at the tumor site. Here, we develop a novel nanoscale coordination polymer using the diblock copolymer poly(2-methacryloyloxyethyl phosphorylcholine)-block-poly(serinyl acrylate) (PMPC-b-PserA) and demonstrate its use for encapsulation of a hydrophobic drug and triggered drug release to induce breast cancer cell apoptosis in vitro. The zwitterionic PMPC block was inspired by the antifouling structure of cell membranes, and the PserA block was inspired by the amphoteric amino acids of proteins. The polymer was synthesized by reversible addition-fragmentation chain transfer polymerization, and a mixture of the polymer and FeCl3 self-assembled into nanoparticles via complexation of Fe3+ with PserA, with the hydrophilic PMPC block at the particle surface. At a molar ratio of Fe3+ to serA of 3:1, the hydrodynamic diameter of the particles was 22.2 nm. Curcumin, a natural water-insoluble polyphenol used to enhance the effects of chemotherapeutics, was encapsulated in the particles as an oil-in-water emulsion, with an encapsulation efficiency of 99.6% and a particle loading capacity of 32%. Triggered release of curcumin was achieved by adding deferoxamine, an FDA-approved Fe3+ chelating agent; curcumin release efficiency increased at higher deferoxamine concentrations and lower pH. Triggered release of curcumin induced apoptosis in human triple-negative breast cancer cells; cell viability decreased to 34.3% after 24 h of treatment with the curcumin-loaded nanoparticles and deferoxamine, versus >80% viability without deferoxamine to trigger drug release. The biocompatibility, tunable composition and size, high hydrophobic drug loading, and triggered-release capability of this nanoscale coordination polymer make it well-suited for use in anticancer drug delivery.
Keywords: anticancer drug delivery; diblock zwitterionic copolymer; nanoscale coordination polymer; polymeric colloid; triggered release.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
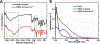
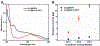
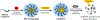
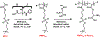
Similar articles
-
Bio-inspired zwitterionic polymeric chelating assembly for treatment of copper-induced cytotoxicity and hemolysis.Mater Sci Eng C Mater Biol Appl. 2021 Oct;129:112367. doi: 10.1016/j.msec.2021.112367. Epub 2021 Aug 20. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34579886
-
Comb-like amphiphilic copolymers bearing acetal-functionalized backbones with the ability of acid-triggered hydrophobic-to-hydrophilic transition as effective nanocarriers for intracellular release of curcumin.Biomacromolecules. 2013 Nov 11;14(11):3973-84. doi: 10.1021/bm401087n. Epub 2013 Oct 29. Biomacromolecules. 2013. PMID: 24107101
-
pH-sensitive micelles self-assembled from polymer brush (PAE-g-cholesterol)-b-PEG-b-(PAE-g-cholesterol) for anticancer drug delivery and controlled release.Int J Nanomedicine. 2017 Mar 21;12:2215-2226. doi: 10.2147/IJN.S130037. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28356738 Free PMC article.
-
Carbohydrate polymer-based nanoparticles in curcumin delivery for cancer therapy.Int J Biol Macromol. 2025 Apr;304(Pt 1):140441. doi: 10.1016/j.ijbiomac.2025.140441. Epub 2025 Jan 28. Int J Biol Macromol. 2025. PMID: 39884595 Review.
-
Solid Lipid Nanoparticles, an Alternative for the Treatment of Triple-Negative Breast Cancer.Int J Mol Sci. 2024 Oct 5;25(19):10712. doi: 10.3390/ijms251910712. Int J Mol Sci. 2024. PMID: 39409041 Free PMC article. Review.
Cited by
-
Applications of Radiolabelled Curcumin and Its Derivatives in Medicinal Chemistry.Int J Mol Sci. 2021 Jul 10;22(14):7410. doi: 10.3390/ijms22147410. Int J Mol Sci. 2021. PMID: 34299029 Free PMC article. Review.
-
Palladium Complex-Supported Silica Nanocarriers: Enhancing Therapeutic Efficacy and Reducing Curcumin Toxicity in Breast Cancer.J Cell Mol Med. 2025 Aug;29(15):e70757. doi: 10.1111/jcmm.70757. J Cell Mol Med. 2025. PMID: 40765065 Free PMC article.
-
Engineering principles of zwitterionic hydrogels: Molecular architecture to manufacturing innovations for advanced healthcare materials.Mater Today Bio. 2025 Jul 12;33:102085. doi: 10.1016/j.mtbio.2025.102085. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40704012 Free PMC article. Review.
-
Current status, challenges and prospects of antifouling materials for oncology applications.Front Oncol. 2024 May 8;14:1391293. doi: 10.3389/fonc.2024.1391293. eCollection 2024. Front Oncol. 2024. PMID: 38779096 Free PMC article. Review.
-
Carbon nanodots constructed by ginsenosides and their high inhibitory effect on neuroblastoma.J Nanobiotechnology. 2023 Jul 28;21(1):244. doi: 10.1186/s12951-023-02023-w. J Nanobiotechnology. 2023. PMID: 37507785 Free PMC article.
References
-
- Lim CK; Singh A; Heo J; Kim D; Lee KE; Jeon H; Koh J; Kwon IC; Kim S Gadolinium-Coordinated Elastic Nanogels for Invivo Tumor Targeting and Imaging. Biomaterials 2013, 34 (28), 6846–6852. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

