Sustained coevolution of phage Lambda and Escherichia coli involves inner- as well as outer-membrane defences and counter-defences
- PMID: 34032565
- PMCID: PMC8290101
- DOI: 10.1099/mic.0.001063
Sustained coevolution of phage Lambda and Escherichia coli involves inner- as well as outer-membrane defences and counter-defences
Abstract
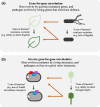
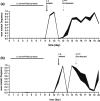
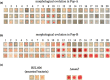
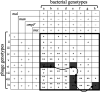
Bacteria often evolve resistance to phage through the loss or modification of cell surface receptors. In Escherichia coli and phage λ, such resistance can catalyze a coevolutionary arms race focused on host and phage structures that interact at the outer membrane. Here, we analyse another facet of this arms race involving interactions at the inner membrane, whereby E. coli evolves mutations in mannose permease-encoding genes manY and manZ that impair λ's ability to eject its DNA into the cytoplasm. We show that these man mutants arose concurrently with the arms race at the outer membrane. We tested the hypothesis that λ evolved an additional counter-defence that allowed them to infect bacteria with deleted man genes. The deletions severely impaired the ancestral λ, but some evolved phage grew well on the deletion mutants, indicating that they regained infectivity by evolving the ability to infect hosts independently of the mannose permease. This coevolutionary arms race fulfils the model of an inverse gene-for-gene infection network. Taken together, the interactions at both the outer and inner membranes reveal that coevolutionary arms races can be richer and more complex than is often appreciated.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

References
-
- Lenski RE, Levin BR. Constraints on the coevolution of bacteria and virulent phage: a model, some experiments, and predictions for natural communities. Am Nat. 1985;125:585–602. doi: 10.1086/284364. - DOI
-
- Chao L, Levin BR, Stewart FM. A complex community in a simple habitat: an experimental study with bacteria and phage. Ecology. 1977;58:369–378. doi: 10.2307/1935611. - DOI
-
- Levin BR, Stewart FM, Chao L. Resource-limited growth, competition, and predation: a model and experimental studies with bacteria and bacteriophage. The American Naturalist. 1977;111:3–24. doi: 10.1086/283134. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

