Placental mTOR complex 1 regulates fetal programming of obesity and insulin resistance in mice
- PMID: 34032632
- PMCID: PMC8410096
- DOI: 10.1172/jci.insight.149271
Placental mTOR complex 1 regulates fetal programming of obesity and insulin resistance in mice
Abstract
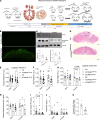
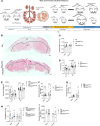
Fetal growth restriction, or low birth weight, is a strong determinant for eventual obesity and type 2 diabetes. Clinical studies suggest placental mechanistic target of rapamycin (mTOR) signaling regulates fetal birth weight and the metabolic health trajectory of the offspring. In the current study, we used a genetic model with loss of placental mTOR function (mTOR-KOPlacenta) to test the direct role of mTOR signaling on birth weight and metabolic health in the adult offspring. mTOR-KOPlacenta animals displayed reduced placental area and total weight, as well as fetal body weight at embryonic day (E) 17.5. Birth weight and serum insulin levels were reduced; however, β cell mass was normal in mTOR-KOPlacenta newborns. Adult mTOR-KOPlacenta offspring, under a metabolic high-fat challenge, displayed exacerbated obesity and metabolic dysfunction compared with littermate controls. Subsequently, we tested whether enhancing placental mTOR complex 1 (mTORC1) signaling, via genetic ablation of TSC2, in utero would improve glucose homeostasis in the offspring. Indeed, increased placental mTORC1 conferred protection from diet-induced obesity in the offspring. In conclusion, placental mTORC1 serves as a mechanistic link between placental function and programming of obesity and insulin resistance in the adult offspring.
Keywords: Diabetes; Endocrinology; Islet cells; Metabolism; Obesity.
Conflict of interest statement
Figures



References
-
- Danaei G, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

