Efficacy and safety of two different adjuvant chemotherapy regimens in combination with concurrent chemoradiotherapy in treating patients with advanced nasopharyngeal carcinoma: A protocol for randomized controlled trial
- PMID: 34032710
- PMCID: PMC8154501
- DOI: 10.1097/MD.0000000000025980
Efficacy and safety of two different adjuvant chemotherapy regimens in combination with concurrent chemoradiotherapy in treating patients with advanced nasopharyngeal carcinoma: A protocol for randomized controlled trial
Abstract
Background: Concurrent chemoradiotherapy is widely utilised as a standardized primary method of treatment for patients with advanced nasopharyngeal carcinoma (NPC). However, the combination of concurrent chemoradiotherapy and adjuvant chemotherapy for treating NPC patients remain unclear. Therefore, this study attempts to elucidate the efficiency and safety of concurrent chemoradiotherapy combined with adjuvant chemotherapy (gemcitabine plus cisplatin versus 5-fluorouracil plus cisplatin) for treating patients with NPC.
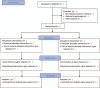
Materials and methods: This study is a randomized, multicentral, open-labelled trial to assess the clinical efficiency and safety of using concurrent chemoradiotherapy combined with adjuvant chemotherapy as a therapeutic measure for advanced NPC patients. A total of 50 patients will be randomly assigned into 2 groups, namely treatment-group-one and treatment-group-two. Eligible patients will be administered with concurrent chemoradiotherapy and subsequentially with adjuvant chemotherapy (gemcitabine plus cisplatin or 5-fluorouracil plus cisplatin). Moreover, the primary endpoint is a comparison of progression-free survival between concurrent chemoradiotherapy and subsequentially adjuvant gemcitabine and cisplatin and chemoradiotherapy, which is proceeded by adjuvant 5-fluorouracil and cisplatin in advanced NPC patients. Overall survival, overall response rate, incidence of acute and late toxicity, and adverse events are the minor endpoints. Statistical analyses will be performed with SPSS 25.0 software.
Discussion: The current research evaluates the clinical efficiency and safety of utilising concurrent chemoradiotherapy combined with adjuvant chemotherapy as a therapeutic strategy to treat advanced NPC patients. The work done in this study will provide a clinical basis for concurrent chemoradiotherapy in combination with adjuvant chemotherapy for treating advanced NPC.
Trial registration: DOI 10.17605/OSF.IO/5UPVM.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interests to disclose.
Similar articles
-
Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial.Lancet Oncol. 2012 Feb;13(2):163-71. doi: 10.1016/S1470-2045(11)70320-5. Epub 2011 Dec 7. Lancet Oncol. 2012. PMID: 22154591 Clinical Trial.
-
Induction chemotherapy with lobaplatin and fluorouracil versus cisplatin and fluorouracil followed by chemoradiotherapy in patients with stage III-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised, controlled, phase 3 trial.Lancet Oncol. 2021 May;22(5):716-726. doi: 10.1016/S1470-2045(21)00075-9. Epub 2021 Apr 12. Lancet Oncol. 2021. PMID: 33857411 Clinical Trial.
-
Raltitrexed versus 5-fluorouracil with cisplatin and concurrent radiotherapy for locally advanced nasopharyngeal carcinoma: An open labeled, randomized, controlled, and multicenter clinical trial.Cancer Med. 2020 Sep;9(17):6166-6172. doi: 10.1002/cam4.3260. Epub 2020 Jul 12. Cancer Med. 2020. PMID: 32657029 Free PMC article. Clinical Trial.
-
Role of chemotherapy in stage IIb nasopharyngeal carcinoma.Chin J Cancer. 2012 Dec;31(12):573-8. doi: 10.5732/cjc.011.10433. Epub 2012 Jul 4. Chin J Cancer. 2012. PMID: 22776232 Free PMC article. Review.
-
Weekly versus triweekly cisplatin treatment in patients with locally advanced nasopharyngeal cancer during concurrent chemoradiotherapy.Eur J Med Res. 2023 Oct 5;28(1):399. doi: 10.1186/s40001-023-01297-y. Eur J Med Res. 2023. PMID: 37794519 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Bensouda Y, Kaikani W, Ahbeddou N, Rahhali R, Jabri M, Mrabti H. Treatment for metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis 2011;128:79–85. - PubMed
-
- Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163–71. - PubMed
-
- Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509–20. - PubMed
-
- Tang LQ, Chen DP, Guo L, Mo HY, Huang Y, Guo SS. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 2018;19:461–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources