Mechanism of p38 MAPK-induced EGFR endocytosis and its crosstalk with ligand-induced pathways
- PMID: 34032851
- PMCID: PMC8155814
- DOI: 10.1083/jcb.202102005
Mechanism of p38 MAPK-induced EGFR endocytosis and its crosstalk with ligand-induced pathways
Abstract
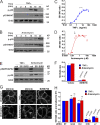
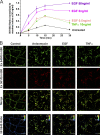
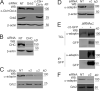
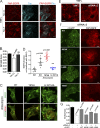
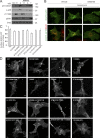
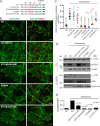
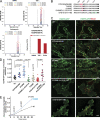
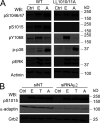
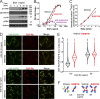
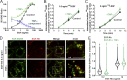
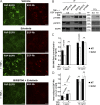
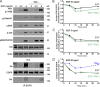
Ligand binding triggers clathrin-mediated and, at high ligand concentrations, clathrin-independent endocytosis of EGFR. Clathrin-mediated endocytosis (CME) of EGFR is also induced by stimuli activating p38 MAPK. Mechanisms of both ligand- and p38-induced endocytosis are not fully understood, and how these pathways intermingle when concurrently activated remains unknown. Here we dissect the mechanisms of p38-induced endocytosis using a pH-sensitive model of endogenous EGFR, which is extracellularly tagged with a fluorogen-activating protein, and propose a unifying model of the crosstalk between multiple EGFR endocytosis pathways. We found that a new locus of p38-dependent phosphorylation in EGFR is essential for the receptor dileucine motif interaction with the σ2 subunit of clathrin adaptor AP2 and concomitant receptor internalization. p38-dependent endocytosis of EGFR induced by cytokines was additive to CME induced by picomolar EGF concentrations but constrained to internalizing ligand-free EGFRs due to Grb2 recruitment by ligand-activated EGFRs. Nanomolar EGF concentrations rerouted EGFR from CME to clathrin-independent endocytosis, primarily by diminishing p38-dependent endocytosis.
© 2021 Perez Verdaguer et al.
Figures

References
-
- Ahmad, G., Mohapatra B.C., Schulte N.A., Nadeau S.A., Luan H., Zutshi N., Tom E., Ortega-Cava C., Tu C., Sanada M., et al. . 2014. Cbl-family ubiquitin ligases and their recruitment of CIN85 are largely dispensable for epidermal growth factor receptor endocytosis. Int. J. Biochem. Cell Biol. 57:123–134. 10.1016/j.biocel.2014.10.019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

