Topical azole treatments for otomycosis
- PMID: 34033120
- PMCID: PMC8147581
- DOI: 10.1002/14651858.CD009289.pub2
Topical azole treatments for otomycosis
Abstract
Background: Otomycosis is a fungal infection of the outer ear, which may be treated with topical antifungal medications. There are many types, with compounds belonging to the azole group ('azoles') being among the most widely used.
Objectives: To evaluate the benefits and harms of topical azole treatments for otomycosis.
Search methods: The Cochrane ENT Information Specialist searched the Cochrane ENT Register; Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The search date was 11 November 2020.
Selection criteria: We included randomised controlled trials (RCTs) in adults and children with otomycosis comparing any topical azole antifungal with: placebo, no treatment, another type of topical azole or the same type of azole but applied in different forms. A minimum follow-up of two weeks was required.
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were: 1) clinical resolution as measured by the proportion of participants with complete resolution at between two and four weeks after treatment (however defined by the authors of the studies) and 2) significant adverse events. Secondary outcomes were 3) mycological resolution and 4) other less serious adverse effects. We used GRADE to assess the certainty of evidence for each outcome.
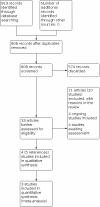
Main results: We included four studies with 559 participants from Spain, Mexico and India. Three studies included children and adults; one included only adults. The duration of symptoms was not always explicitly stated. Mycological resolution results were only reported in one study. The studies assessed two comparisons: one type of topical azole versus another and the same azole but administered in different forms (cream versus solution). A. Topical azoles versus placebo None of the studies assessed this comparison. B. Topical azoles versus no treatment None of the studies assessed this comparison. C. One type of topical azole versus another type of topical azole i) Clotrimazole versus other types of azoles (eberconazole, fluconazole, miconazole) Three studies examined clotrimazole versus other types of azoles. The evidence is very uncertain about the difference between clotrimazole and other types of azole in achieving complete clinical resolution at four weeks (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.59 to 1.07; 3 studies; 439 participants; very low-certainty evidence). The anticipated absolute effects are 668 per 1000 for clotrimazole versus 835 per 1000 for other azoles. One study planned a safety analysis and reported no significant adverse events in either group. The evidence is therefore very uncertain about any differences between clotrimazole and other types of azole (no events in either group; 1 study; 174 participants; very low-certainty evidence). Clotrimazole may result in little or no difference in mycological resolution at two weeks follow-up (RR 1.01, 95% CI 0.96 to 1.06; 1 study; 174 participants; low-certainty evidence) or in other (less serious) adverse events at two weeks follow-up (36 per 1000, compared to 45 per 1000, RR 0.79, 95% CI 0.18 to 3.41; 1 study; 174 participants; very low-certainty evidence). ii) Bifonazole cream versus bifonazole solution One study compared bifonazole 1% cream with solution. Bifonazole cream may have little or no effect on clinical resolution at two weeks follow-up when compared to solution, but the evidence is very uncertain (RR 1.07, 95% CI 0.73 to 1.57; 1 study; 40 ears; very low-certainty evidence). Bifonazole cream may achieve less mycological resolution compared to solution at two weeks after the end of therapy, but the evidence for this is also very uncertain (RR 0.53, 95% CI 0.29 to 0.96; 1 study; 40 ears; very low-certainty evidence). Five out of 35 patients sustained severe itching and burning from the bifonazole solution but none with the bifonazole cream (very low-certainty evidence).
Authors' conclusions: We found no studies that evaluated topical azoles compared to placebo or no treatment. The evidence is very uncertain about the effect of clotrimazole on clinical resolution of otomycosis, on significant adverse events or other (non-serious) adverse events when compared with other topical azoles (eberconazole, fluconazole, miconazole). There may be little or no difference between clotrimazole and other azoles in terms of mycological resolution. It may be difficult to generalise these results because the range of ethnic backgrounds of the participants in the studies is limited.
Trial registration: ClinicalTrials.gov NCT01993823.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Ambrose Lee: none known.
James Tysome: none known.
Shakeel Saeed: none known.
Figures



Update of
- doi: 10.1002/14651858.CD009289
References
References to studies included in this review
de la Paz Cota 2018 {published data only}
-
- la Paz Cota B, Vegas P, Navarrete J, Mulgado G, Huerta J, Bautista E, et al. Efficacy and safety of eberconazole 1% otic solution compared to clotrimazole 1% solution in patients with otomycosis. American Journal of Otolaryngology 2018;39:307-12. - PubMed
-
- NCT01993823. A phase III, multicenter, randomized, double-blind, parallel group, active treatment-controlled study assessing the safety and efficacy of G238 compared to clotrimazole 1% otic solution in patients with otomycosis. http://clinicaltrials.gov/show/NCT01993823 (first received 25 November 2013). [4291136]
del Palacio 1993 {published data only}
-
- Palacio A, Lopez-Suso M, Moore M, Cuetara M, Garcia-La Calle C, Noriega A. Long term follow-up of otomycosis and its treatment with bifonazole. Journal of Medical and Veterinary Mycology 1993;31:435-47.
Navaneethan 2014 {published data only}
Prabhakaran 2018 {published data only}
-
- Prabhakaran P, Navin N, Srinivasan R, Palanisamy T, Kanagamuthu P, Somu P, et al. A comparative study of efficacy of clotrimazole and fluconazole ear drops in otomycosis. Journal of Evolution of Medical and Dental Sciences 2018;7(17):2058-61. [DOI: ]
References to studies excluded from this review
Albano 1966 {published data only}
-
- Albano V. Contribution to the therapy of otomycosis. Annali di Laringologia, Otologia, Rinologia, Faringologia 1966;65(4):521-9. [CRS:10899693] - PubMed
Beck 1969 {published data only}
-
- Beck C. Contribution to the therapy of otomycosis. Zeitschrift fur Laryngologie, Rhinologie, Otologie und ihre Grenzgebiete 1969;48(2):119-23. [CRS:10899683] - PubMed
Berjis 2012 {published data only}
-
- Berjis N, Okhovat S, Koujani Z, Baradaran S. Comparing the therapeutic effect of clotrimazole and tolnaftate in treating variety of fungal species producing otomycosis in Alzahra and Kashani Hospitals, Iran. Journal of Isfahan Medical School 2012;29:164. [CRS:10899759.]
Boncalon 2009 {published data only}
-
- Boncalon R, Arguay M, Ramos R. A preliminary study on the efficacy of Plumeria acuminata (Kalachuchi) bark extract ointment versus clotrimazole cream in the treatment of otomycosis. Philippine Journal of Otolaryngology Head and Neck Surgery 2009;24(1):5-8. [CRS: 10899322.]
Carrat 2001 {published data only}
-
- Carrat X, Bordure P, Dutronc H, Lacher G, Malard O. Otomycosis. Revue de Laryngologie - Otologie - Rhinologie 2001;122(2):137-43. [CRS:10899567] - PubMed
Cojocaru 1970 {published data only}
-
- Cojocaru I, Alteras I, Dulamita L. Some data on the treatment of otomycoses. Mykosen 1970;13(5):243-6. [CRS: 10899680] - PubMed
del Palacio 2002 {published data only}
-
- Palacio A, Cuetara MS, Lopez-Suso MJ, Amor E, Garau M. Randomized prospective comparative study: short-term treatment with ciclopiroxolamine (cream and solution) versus boric acid in the treatment of otomycosis. Mycoses 2002;45(8):317-28. [CRS: 10899300.] - PubMed
Diaz‐Pavon 2018 {unpublished data only}
-
- Diaz-Pavon GA, Jimenez-Garcia L, Munoz-Estrada VF, Aguilar EC. Efficacy of clotrimazole versus tolnaftate in otomycosis treatment. Otolaryngology - Head and Neck Surgery 2018;159 Suppl 1:265-6. [CRS:10899491]
Gordana 2007 {published data only}
-
- Gordana R, Mirko P, Radjenovic G, Popovic M. Castellani tintura rubra versus dexamethasone neomycin; an effective cure for external otitis. European Archives of Oto-rhino-laryngology 2007;264(Suppl 1):S153-264. [CRS: 4146776]
Jimenez‐Garcia 2019 {published data only}
-
- Jimenez-Garcia L, Celis-Aguilar E, Diaz-Pavon G, Munoz Estrada V, Castro-Urquizo A, Hernandez-Castillo N, et al. Efficacy of topical clotrimazole vs. topical tolnaftate in the treatment of otomycosis. A randomized controlled clinical trial. Brazilian Journal of Otorhinolaryngology 2020;86(3):300-7. [PMID: ] - PMC - PubMed
Joiemon 2006 {published data only}
-
- Joeimon J, Dhinesh Babu K. Open randomized clinical study on otomycosis. Indian Journal of Pharmacology 2006;38(1):86. [CRS: 10899319]
Khamaganova 2010 {published data only}
-
- Khamaganova I, Piven' NP. Combined treatment of external auditory canal diseases in dermatological practice. Vestnik Otorinolaringologii 2010;2:66-8. [CRS: 10899458] [PMID: ] - PubMed
Malik 2012 {published data only}
-
- Malik A, Malik S, Aslam M. Comparative efficacy of topical clotrimazole and 3% salicylic acid in otomycosis. Rawal Medical Journal 2012;37(1):1-10.
Mofatteh 2017 {published data only}
Nunez Navarrete 2000 {published data only}
-
- Nunez Navarrete E, Consuelo Diaz Elizalde N. Otomycosis. Two therapeutic approaches [Otitis externa micotica. Dos esquemas terapeuticos]. Revista Médica del IMSS 2000;38(6):467-72.
Pal'chun 2012 {published data only}
-
- Pal'chun V, Ogorodnikov D. The experience with the supervision and treatment of the patients presenting with external otitis. Vestnik Otorinolaringologii 2012;2:53-6. [CRS: 10899420] - PubMed
Rafique 2014 {published data only}
-
- Rafique M, Udaipurwala I, Ehsan U. Suction cleaning of the external auditory canal in otomycosis: is it really helpful? Journal of the Liaquat University of Medical and Health Sciences 2014;13(3):97-100. [CRS:10899341]
Romsaithong 2016 {published data only}
-
- NCT01547221. Effectiveness of 3% boric acid in isopropyl alcohol versus 1% clotrimazole solution in otomycosis patients: a randomized controlled trial. http://clinicaltrials.gov/show/NCT01547221 (first received 7 March 2012). [CENTRAL: CN-00855127]
-
- Romsaithong S, Tomanakan K, Tangsawad W, Thanaviratananich S. Effectiveness of 3 per cent boric acid in 70 per cent alcohol versus 1 per cent clotrimazole solution in otomycosis patients: a randomised, controlled trial. Journal of Laryngology & Otology 2016;130(9):811-5. - PubMed
Wei 2018 {published data only}
-
- Wei XM, Lu L, Gao X. Effect observation of cleaning up the external auditory canal by otoendoscope combined with clotrimazole ointment in the treatment of pregnancy with otitis externa mycotica. Lin Chuang Er Bi Yan Hou Ke za Zhi [Journal of Clinical Otorhinolaryngology] 2018;32(2):134-7. [CRS:10899484] - PubMed
Zhang 2010 {published data only}
-
- Zhang H, Gu L. Effect of fluconazole eye drops on otomycosis external. Practical Pharmacy and Clinical Remedies 2010;13(6):461-2. [CRS: 10899325]
References to studies awaiting assessment
Bambule 1978 {published data only}
-
- Bambule J, Grigoriu D. Otomycoses and their treatment. Mykosen. Supplement 1978;1:82-6. [CRS:10899662.] - PubMed
Ben Rejeb 1984 {published data only}
-
- Ben Rejeb A, Jelloul N, Kchir N, Bouzaiane A, Zitouna MM, Ben Rachid MS, et al. Otomycosis. Tunisie Medicale 1984;62(5):381-4. [CRS:10899643.] - PubMed
Hu 2007 {published data only}
-
- Hu C, Zhang J, Min X. Efficacy of fluconazole ear drops in otomycosis external. West China Medical Journal 2007;4:805-6. [CRS: 10899320]
Ren 2015 {published data only}
-
- Ren Y, Zhang Q, Yu Z. Clinical analysis of external ear canal coating by otoendoscopy on otomycosis external with triamcinolone acetonide and econazole nitrate cream. Lin Chuang Er Bi Yan Hou Ke za Zhi [Journal of Clinical Otorhinolaryngology] 2015;29(14):1304-5. [CRS: 10899361] - PubMed
References to ongoing studies
IRCT201502241138N15 {published and unpublished data}
-
- IRCT201502241138N15. Efficacy of clotrimazole 1% and miconazole 1% in otomycosis. https://en.irct.ir/trial/187 (first received 26 March 2015). [CRS: 10899510]
IRCT2015040513136N3 {unpublished data only}
-
- IRCT2015040513136N3. The effect of clotrimazole 1% on recurrence of otomycosis after treatment. http://en.irct.ir/trial/13057 (first received 9 February 2016). [CRS:10899745]
IRCT2016082313136N4 {unpublished data only}
-
- IRCT2016082313136N4. The effect clotrimazole ointment comparison to ceftizoxime powder and clotrimazole ointment in the treatment of patients with fungal otitis media. https://en.irct.ir/trial/13058 (first received 13 October 2016). [CRS:10899506]
NCT03686397 {unpublished data only}
-
- EUCTR2019-003463-22. Clinical trial to assess the efficacy and safety of clotrimazole 1% otic solution compared to placebo for the treatment of fungal otitis externa (otomycosis) [A phase III, multicenter, randomized, double-blind clinical trial to assess the efficacy and safety of clotrimazole 1% otic solution compared to placebo for the treatment of fungal otitis externa (otomycosis)- CLEAR 2]. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_nu... (first received 16 January 2020).
-
- NCT03686397. SVT-15652 otic solution for the treatment of otomycosis [A phase III, multicenter, randomized, double-blind clinical trial to assess the efficacy and safety of SVT-15652 otic solution complared to placebo for the treatment of fungal otitis externa (otomycosis)]. https://clinicaltrials.gov/ct2/show/NCT03686397 (first received 26 September 2018). [CENTRAL: CN-01648794]
Additional references
Arndal 2016
-
- Arndal E, Glad H, Homoe P. Large discrepancies in otomycosis treatment in private ear, nose and throat clinic in Denmark. Danish Medical Journal 2016;63(5):1-5. - PubMed
Bassiouny 1986
-
- Bassiouny A, Kamel T, Moawad M, Hindawy D. Broad spectrum antifungal agents in otomycosis. Journal of Laryngology and Otology 1986;100:867-73. - PubMed
Bhutta 2020
Blyth 2007
-
- Blyth C, Palasanthiran P, O'Brien T. Antifungal therapy in children with invasive fungal infections: a systematic review. Pediatrics 2007;119:772-84. - PubMed
Blyth 2011
-
- Blyth C, Gomes L, Sorrell T, da Cruz M, Sud A, Chen SC. Skull-base osteomyelitis: fungal vs. bacterial infection. Clinical Microbiology and Infection 2011;17(2):306-11. - PubMed
CONSORT 2010
Cota 2018
-
- la Paz Cota B, Vega P, Navarrete J, Mulgado G, Huerta J, Bautista E, et al. Efficacy and safety of eberconazole 1% otic solution compared to clotrimazole 1% in patients with otomycosis. American Journal of Otolaryngology 2018;39:307-12. - PubMed
Enweani 1998
-
- Enweani I, Igumbor H. Prevalence of otomycosis in malnourished children in Edo State, Nigeria. Mycopathologia 1998;140:85-7. - PubMed
Gharaghani 2015
-
- Gharaghani M, Seifi Z, Zarei Mahmoudabadi A. Otomycosis in Iran: a review. Mycopathologia 2015;179(5-6):415-24. - PubMed
Hamza 2011
-
- Hamza A, Khan Q, Khan M. Efficacy of topical clotrimazole in otomycosis. Pacific Journal of Medical and Health Sciences 2011;5(4):738-40.
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Head 2020
Herasym 2016
-
- Herasym K, Bonaparte J, Kilty S. A comparison of Locacorten-Vioform and clotrimazole in otomycosis: a systematic review and one-way meta-analysis. Laryngoscope 2016;126:1411-9. - PubMed
Ho 2006
-
- Ho T, Vrabec JT, Yoo D, Coker NJ. Otomycosis: clinical features and treatment implications. Otolaryngology - Head and Neck Surgery 2006;135(5):787-91. - PubMed
Isaacson 2020
-
- Isaacson G. Oxymetazoline, mupirocin, clotrimazole—safe, effective, off-label agents for tympanostomy tube care. Ear, Nose & Throat Journal 2020;99(1 Suppl):30S-4S. - PubMed
Jackman 2005
-
- Jackman A, Ward R, April M, Bent J. Topical antibiotic induced otomycosis. International Journal of Pediatric Otolaryngology 2005;69(6):857-60. - PubMed
Jadhav 2003
-
- Jadhav VJ, Pal M, Mishra GS. Etiological significance of Candida albicans in otitis externa. Mycopathologia 2003;156:313-5. - PubMed
Kaushik 2010
Khan 2013
-
- Khan F, Muhammad R, Muhammad RK, Rehman F, Iqbal J, Khan M, et al. Efficacy of topical clotrimazole in treatment of otomycosis. Journal of Ayub Medical College, Abbottabad 2013;25(1-2):78-80. - PubMed
Kurnatowski 2001
-
- Kurnatowski P, Filipiak A. Otomycosis: prevalence, clinical symptoms, therapeutic procedures. Mycoses 2001;44:472-9. - PubMed
MedDRA
-
- Medical Dictionary for Regulatory Activities. http://meddra.org.
Mofatteh 2018
Munguia 2008
-
- Munguia R, Daniel S. Ototopical antifungals and otomycosis: a review. International Journal of Pediatric Otolaryngology 2008;72(4):453-9. - PubMed
NCT01547221
-
- NCT01547221. Effectiveness of 3% boric acid in 70% alcohol versus 1% clotrimazole solution in otomycosis patients. https://clinicaltrials.gov/show/NCT01547221 (first received 7 March 2012).
Pradhan 2003
-
- Pradhan B, Tuladhar N, Amatya R. Prevalence of otomycosis in outpatient Department of Otolaryngology in Tribhuvan University Teaching Hospital, Kathmandu, Nepal. Annals of Otology, Rhinology and Laryngology 2003;112:384-7. - PubMed
RevMan 2014 [Computer program]
-
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowlands 2001
Sheehan 1999
Stern 1988
-
- Stern J, Shah M, Lucente F. In vitro effectiveness of 13 agents in otomycosis and review of the literature. Laryngoscope 1988;98:1173-7. - PubMed
Vennewald 2010
-
- Vennewald I, Klemm E. Otomycosis: diagnosis and treatment. Clinics in Dermatology 2010;28(2):202-11. - PubMed
Walsh 2008
-
- Walsh T, Annissie E, Denning D, Herbrecht R, Kontoyiannis D, Marr K, et al. Treatment of aspergillosis: Clinical Practice Guidelines of the Infectious Disease Society of America. Clinical Infectious Diseases 2008;46:327-60. - PubMed
WHO 2006
-
- World Health Organization. Primary Ear and Hearing Care Training Resource: Advanced Level. Geneva: World Health Organization, 2006.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

