Community-based smoking cessation treatment for adults with high anxiety sensitivity: a randomized clinical trial
- PMID: 34033178
- PMCID: PMC10091508
- DOI: 10.1111/add.15586
Community-based smoking cessation treatment for adults with high anxiety sensitivity: a randomized clinical trial
Abstract
Background and aims: People with anxiety disorders are more likely to smoke and less likely to succeed when they try to quit. Anxiety sensitivity may underlie both phenomena, such that people with high anxiety sensitivity react to interoceptive distress by avoidance. This study aimed to test the efficacy of an exercise program that induced interoceptive distress and thereby created tolerance to this distress in a safe environment.
Design, setting and participants: Randomized clinical trial at four YMCA branches in Austin, Texas, USA. Participants [n = 150; 130 (86.7%) white; 101 (67.3%) female; meanage = 38.6, standard deviation (SD)age = 10.4] were adult, daily smokers with high anxiety sensitivity motivated to quit smoking, who reported no regular moderate-intensity exercise.
Interventions: Participants were assigned a YMCA personal trainer who guided them through a 15-week intervention aerobic exercise program. Participants assigned to the personalized intervention trained at 60-85% of their heart rate reserve (HRR), whereas participants assigned to the control intervention trained at 20-40% of their HRR. Participants in both groups received standard behavioral support and nicotine replacement therapy.
Measurements: The primary outcome was biologically verified 7-day point prevalence abstinence (PPA) at 6-month follow-up.
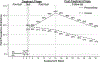
Findings: Sixty-one per cent of participants were available at the 6-month follow-up. PPA at 6 months was higher in the personalized intervention than the control intervention [27.6 versus 14.8%; odds ratio (OR) = 2.20, 95% confidence interval (CI) = 1.28, 3.80, P = 0.005], assuming missing at random. Anxiety sensitivity declined in both groups with no evidence that this differed between groups.
Conclusions: An exercise program of high intensity increased abstinence from smoking in people with high anxiety sensitivity, but may not have done so by reducing anxiety sensitivity.
Trial registration: ClinicalTrials.gov NCT03080090.
Keywords: Anxiety; anxiety sensitivity; community-based intervention; exercise; smoking cessation; tobacco quitline.
© 2021 Society for the Study of Addiction.
Conflict of interest statement
Figures
References
-
- Substance Abuse and Mental Health Services Administration (US), Office of the Surgeon General (US). Smoking Cessation: A Report of the Surgeon General [Internet]. Washington (DC): US Department of Health and Human Services; 2020. [cited 2020 Sep 25]. (Publications and Reports of the Surgeon General). Available from: http://www.ncbi.nlm.nih.gov/books/NBK555591/
-
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky MJ, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10:1691–715. - PubMed
-
- Kearns NT, Carl E, Stein AT, Vujanovic AA, Zvolensky MJ, Smits JAJ, et al. Posttraumatic stress disorder and cigarette smoking: A systematic review. Depress Anxiety. 2018;35(11):1056–72. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical