Pregabalin as adjunctive therapy in adult and pediatric patients with generalized tonic-clonic seizures: A randomized, placebo-controlled trial
- PMID: 34033265
- PMCID: PMC8166786
- DOI: 10.1002/epi4.12492
Pregabalin as adjunctive therapy in adult and pediatric patients with generalized tonic-clonic seizures: A randomized, placebo-controlled trial
Abstract
Objective: Generalized tonic-clonic (GTC) seizures are the most common type of generalized seizure and more common in children than adults. This phase 3 study evaluated the efficacy and safety of pregabalin for GTC seizures in adults and children with epilepsy.
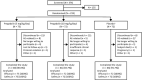
Methods: This randomized, double-blind, multicenter study evaluated pregabalin (5 mg/kg/day or 10 mg/kg/day) vs placebo as adjunctive therapy for 10 weeks (following a 2-week dose escalation), in pediatric and adult patients (aged 5-65 years) with GTC seizures. Primary endpoint was change in log-transformed 28-day seizure rate during active treatment. Secondary endpoints included responder rates, defined as proportion of patients with ≥50% reduction in 28-day GTC seizure rate from baseline. Safety was monitored throughout.
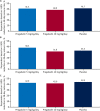
Results: Of 219 patients, 75, 72, and 72 were randomized to adjunctive pregabalin 5 mg/kg/day, 10 mg/kg/day, and placebo, respectively. Fifteen, 11, and 6 patients discontinued from the 5 mg/kg/day, 10 mg/kg/day, and placebo arms, respectively, most commonly due to adverse events (AEs; 10.7%, 6.9%, and 5.6%, respectively). A nonsignificant change in log-transformed mean 28-day seizure rate was seen with pregabalin 10 mg/kg/day vs placebo (least-squares [LS] mean difference -0.01 [95% confidence interval (CI) -0.19 to 0.16]; P = .8889) and with pregabalin 5 mg/kg/day vs placebo (LS mean difference 0.02 [CI -0.15 to 0.19]; P = .8121). Similar observations were noted for adults and children. No significant differences were seen for secondary endpoints with pregabalin vs placebo, including responder rate. The most common AEs (≥10%) were dizziness, headache, and somnolence. Most were of mild/moderate intensity. Seven patients had serious AEs, with one death in the placebo arm (sudden unexpected death in epilepsy).
Significance: Adjunctive pregabalin treatment did not change GTC seizure rate in adults or children. The safety profile of pregabalin was similar to that known; treatment was well tolerated with few discontinuations due to AEs.
Trial registration: ClinicalTrials.gov NCT01747915.
Keywords: antiepileptic drug; epilepsy; generalized tonic-clonic seizures; pregabalin.
© 2021 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
J Driscoll, M Almas, G Gregorian, J Liu, J Patrick, and J Antinew are full‐time employees of Pfizer. JM Scavone was a full‐time employee of Pfizer at the time of study conduct. A Kyrychenko has no conflict of interest to report. I Makedonska has no conflict of interest to report.
Figures

Similar articles
-
Lamotrigine adjunctive therapy among children and adolescents with primary generalized tonic-clonic seizures.Pediatrics. 2006 Aug;118(2):e371-8. doi: 10.1542/peds.2006-0148. Epub 2006 Jul 17. Pediatrics. 2006. PMID: 16847080 Clinical Trial.
-
Pregabalin adjunctive therapy for focal onset seizures in children 1 month to <4 years of age: A double-blind, placebo-controlled, video-electroencephalographic trial.Epilepsia. 2020 Apr;61(4):617-626. doi: 10.1111/epi.16466. Epub 2020 Mar 18. Epilepsia. 2020. PMID: 32189338 Clinical Trial.
-
Pregabalin as Adjunctive Treatment for Focal Onset Seizures in Pediatric Patients: A Randomized Controlled Trial.J Child Neurol. 2019 Apr;34(5):248-255. doi: 10.1177/0883073818821035. Epub 2019 Jan 27. J Child Neurol. 2019. PMID: 30688135 Clinical Trial.
-
Perampanel as monotherapy and adjunctive therapy for focal onset seizures, focal to bilateral tonic-clonic seizures and as adjunctive therapy of generalized onset tonic-clonic seizures.Expert Rev Neurother. 2019 Jan;19(1):5-16. doi: 10.1080/14737175.2019.1555474. Epub 2018 Dec 18. Expert Rev Neurother. 2019. PMID: 30560703 Review.
-
Topiramate versus carbamazepine monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2019 Jun 24;6(6):CD012065. doi: 10.1002/14651858.CD012065.pub3. Cochrane Database Syst Rev. 2019. PMID: 31233229 Free PMC article.
Cited by
-
Pregabalin Add-On vs. Dose Increase in Levetiracetam Add-On Treatment: A Real-Life Trial in Dogs With Drug-Resistant Epilepsy.Front Vet Sci. 2022 Jul 6;9:910038. doi: 10.3389/fvets.2022.910038. eCollection 2022. Front Vet Sci. 2022. PMID: 35873699 Free PMC article.
-
Efficacy and Safety of Single-Dose Pregabalin in Preoperative Pediatric Sedation.J Pharm Bioallied Sci. 2024 Feb;16(Suppl 1):S901-S904. doi: 10.4103/jpbs.jpbs_1086_23. Epub 2024 Feb 29. J Pharm Bioallied Sci. 2024. PMID: 38595464 Free PMC article.
-
Changes in routine blood parameters of patients with generalized tonic clonic seizure: a retrospective study.Neurosciences (Riyadh). 2023 Apr;28(2):123-129. doi: 10.17712/nsj.2023.2.20220135. Neurosciences (Riyadh). 2023. PMID: 37045465 Free PMC article.
-
Antiseizure medications for idiopathic generalized epilepsies: a systematic review and network meta-analysis.J Neurol. 2023 Oct;270(10):4713-4728. doi: 10.1007/s00415-023-11834-8. Epub 2023 Jun 28. J Neurol. 2023. PMID: 37378757 Free PMC article.
References
-
- World Health Organization. Epilepsy: a public health imperative . 2019 5 July, 2019. Available from: https://www.who.int/mental_health/neurology/epilepsy/report_2019/en/
-
- Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2019;18:1–7. - PubMed
-
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–30. - PubMed
-
- Panayiotopoulos CP. The new ILAE report on terminology and concepts for organization of epileptic seizures: a clinician's critical view and contribution. Epilepsia. 2011;52(12):2155–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

