Pregabalin as adjunctive therapy in adult and pediatric patients with generalized tonic-clonic seizures: A randomized, placebo-controlled trial
- PMID: 34033265
- PMCID: PMC8166786
- DOI: 10.1002/epi4.12492
Pregabalin as adjunctive therapy in adult and pediatric patients with generalized tonic-clonic seizures: A randomized, placebo-controlled trial
Abstract
Objective: Generalized tonic-clonic (GTC) seizures are the most common type of generalized seizure and more common in children than adults. This phase 3 study evaluated the efficacy and safety of pregabalin for GTC seizures in adults and children with epilepsy.
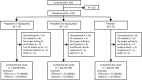
Methods: This randomized, double-blind, multicenter study evaluated pregabalin (5 mg/kg/day or 10 mg/kg/day) vs placebo as adjunctive therapy for 10 weeks (following a 2-week dose escalation), in pediatric and adult patients (aged 5-65 years) with GTC seizures. Primary endpoint was change in log-transformed 28-day seizure rate during active treatment. Secondary endpoints included responder rates, defined as proportion of patients with ≥50% reduction in 28-day GTC seizure rate from baseline. Safety was monitored throughout.
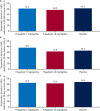
Results: Of 219 patients, 75, 72, and 72 were randomized to adjunctive pregabalin 5 mg/kg/day, 10 mg/kg/day, and placebo, respectively. Fifteen, 11, and 6 patients discontinued from the 5 mg/kg/day, 10 mg/kg/day, and placebo arms, respectively, most commonly due to adverse events (AEs; 10.7%, 6.9%, and 5.6%, respectively). A nonsignificant change in log-transformed mean 28-day seizure rate was seen with pregabalin 10 mg/kg/day vs placebo (least-squares [LS] mean difference -0.01 [95% confidence interval (CI) -0.19 to 0.16]; P = .8889) and with pregabalin 5 mg/kg/day vs placebo (LS mean difference 0.02 [CI -0.15 to 0.19]; P = .8121). Similar observations were noted for adults and children. No significant differences were seen for secondary endpoints with pregabalin vs placebo, including responder rate. The most common AEs (≥10%) were dizziness, headache, and somnolence. Most were of mild/moderate intensity. Seven patients had serious AEs, with one death in the placebo arm (sudden unexpected death in epilepsy).
Significance: Adjunctive pregabalin treatment did not change GTC seizure rate in adults or children. The safety profile of pregabalin was similar to that known; treatment was well tolerated with few discontinuations due to AEs.
Trial registration: ClinicalTrials.gov NCT01747915.
Keywords: antiepileptic drug; epilepsy; generalized tonic-clonic seizures; pregabalin.
© 2021 The Authors. Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
J Driscoll, M Almas, G Gregorian, J Liu, J Patrick, and J Antinew are full‐time employees of Pfizer. JM Scavone was a full‐time employee of Pfizer at the time of study conduct. A Kyrychenko has no conflict of interest to report. I Makedonska has no conflict of interest to report.
Figures

References
-
- World Health Organization. Epilepsy: a public health imperative . 2019 5 July, 2019. Available from: https://www.who.int/mental_health/neurology/epilepsy/report_2019/en/
-
- Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2019;18:1–7. - PubMed
-
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–30. - PubMed
-
- Panayiotopoulos CP. The new ILAE report on terminology and concepts for organization of epileptic seizures: a clinician's critical view and contribution. Epilepsia. 2011;52(12):2155–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

