Metformin: Experimental and Clinical Evidence for a Potential Role in Emphysema Treatment
- PMID: 34033525
- PMCID: PMC8521702
- DOI: 10.1164/rccm.202012-4510OC
Metformin: Experimental and Clinical Evidence for a Potential Role in Emphysema Treatment
Abstract
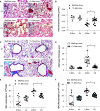
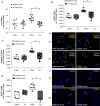
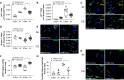
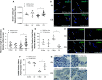
Rationale: Cigarette smoke (CS) inhalation triggers oxidative stress and inflammation, leading to accelerated lung aging, apoptosis, and emphysema, as well as systemic pathologies. Metformin is beneficial for protecting against aging-related diseases. Objectives: We sought to investigate whether metformin may ameliorate CS-induced pathologies of emphysematous chronic obstructive pulmonary disease (COPD). Methods: Mice were exposed chronically to CS and fed metformin-enriched chow for the second half of exposure. Lung, kidney, and muscle pathologies, lung proteostasis, endoplasmic reticulum (ER) stress, mitochondrial function, and mediators of metformin effects in vivo and/or in vitro were studied. We evaluated the association of metformin use with indices of emphysema progression over 5 years of follow-up among the COPDGene (Genetic Epidemiology of COPD) study participants. The association of metformin use with the percentage of emphysema and adjusted lung density was estimated by using a linear mixed model. Measurements and Main Results: Metformin protected against CS-induced pulmonary inflammation and airspace enlargement; small airway remodeling, glomerular shrinkage, oxidative stress, apoptosis, telomere damage, aging, dysmetabolism in vivo and in vitro; and ER stress. The AMPK (AMP-activated protein kinase) pathway was central to metformin's protective action. Within COPDGene, participants receiving metformin compared with those not receiving it had a slower progression of emphysema (-0.92%; 95% confidence interval [CI], -1.7% to -0.14%; P = 0.02) and a slower adjusted lung density decrease (2.2 g/L; 95% CI, 0.43 to 4.0 g/L; P = 0.01). Conclusions: Metformin protected against CS-induced lung, renal, and muscle injury; mitochondrial dysfunction; and unfolded protein responses and ER stress in mice. In humans, metformin use was associated with lesser emphysema progression over time. Our results provide a rationale for clinical trials testing the efficacy of metformin in limiting emphysema progression and its systemic consequences.
Keywords: aging; chronic obstructive pulmonary disease; cigarette smoke; comorbidities; metformin.
Figures


Comment in
-
Can Metformin Downshift the Gears of Aging to Slow Emphysema Progression?Am J Respir Crit Care Med. 2021 Sep 15;204(6):621-622. doi: 10.1164/rccm.202105-1273ED. Am J Respir Crit Care Med. 2021. PMID: 34191690 Free PMC article. No abstract available.
References
-
- Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1257–1266. - PubMed
-
- Celli BR, Locantore N, Tal-Singer R, Riley J, Miller B, Vestbo J, et al. ECLIPSE Study Investigators. Emphysema and extrapulmonary tissue loss in COPD: a multi-organ loss of tissue phenotype. Eur Respir J. 2018;51:1702146. - PubMed
-
- Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, et al. BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:155–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 ES007091/ES/NIEHS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- R01 HL142769/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- R01 HL155948/HL/NHLBI NIH HHS/United States
- P50 ES018176/ES/NIEHS NIH HHS/United States
- K08 HL135402/HL/NHLBI NIH HHS/United States
- P50 MD010431/MD/NIMHD NIH HHS/United States
- R01 HL139617/HL/NHLBI NIH HHS/United States
- R01 HL149744/HL/NHLBI NIH HHS/United States
- R01 HL132523/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

