Cytoskeletal regulation guides neuronal trafficking to effectively supply the synapse
- PMID: 34033795
- PMCID: PMC8360495
- DOI: 10.1016/j.cub.2021.02.024
Cytoskeletal regulation guides neuronal trafficking to effectively supply the synapse
Abstract
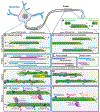
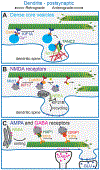
The development and proper function of the brain requires the formation of highly complex neuronal circuitry. These circuits are shaped from synaptic connections between neurons and must be maintained over a lifetime. The formation and continued maintenance of synapses requires accurate trafficking of presynaptic and postsynaptic components along the axon and dendrite, respectively, necessitating deliberate and specialized delivery strategies to replenish essential synaptic components. Maintenance of synaptic transmission also requires readily accessible energy stores, produced in part by localized mitochondria, that are tightly regulated with activity level. In this review, we focus on recent developments in our understanding of the cytoskeletal environment of axons and dendrites, examining how local regulation of cytoskeletal dynamics and organelle trafficking promotes synapse-specific delivery and plasticity. These new insights shed light on the complex and coordinated role that cytoskeletal elements play in establishing and maintaining neuronal circuitry.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJG, Nyengaard JR, and Regeur L (2003). Aging and the human neocortex. Exp. Gerontol. 38, 95–99. - PubMed
-
- Laßek M, Weingarten J, and Volknandt W (2015). The synaptic proteome. Cell Tissue Res. 359, 255–265. - PubMed
-
- Takamori S (2009). Synaptic Vesicles. Encycl. Neurosci, 801–808.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

