A randomized pilot clinical trial of pravastatin versus placebo in pregnant patients at high risk of preeclampsia
- PMID: 34033812
- PMCID: PMC8611118
- DOI: 10.1016/j.ajog.2021.05.018
A randomized pilot clinical trial of pravastatin versus placebo in pregnant patients at high risk of preeclampsia
Abstract
Background: Preeclampsia remains a major cause of maternal and neonatal morbidity and mortality. Biologic plausibility, compelling preliminary data, and a pilot clinical trial support the safety and utility of pravastatin for the prevention of preeclampsia.
Objective: We previously reported the results of a phase I clinical trial using a low dose (10 mg) of pravastatin in high-risk pregnant women. Here, we report a follow-up, randomized trial of 20 mg pravastatin versus placebo among pregnant women with previous preeclampsia who required delivery before 34+6 weeks' gestation with the objective of evaluating the safety and pharmacokinetic parameters of pravastatin.
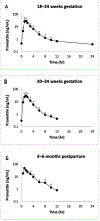
Study design: This was a pilot, multicenter, blinded, placebo-controlled, randomized trial of women with singleton, nonanomalous pregnancies at high risk for preeclampsia. Women between 12+0 and 16+6 weeks of gestation were assigned to receive a daily pravastatin dose of 20 mg or placebo orally until delivery. In addition, steady-state pravastatin pharmacokinetic studies were conducted in the second and third trimesters of pregnancy and at 4 to 6 months postpartum. Primary outcomes included maternal-fetal safety and pharmacokinetic parameters of pravastatin during pregnancy. Secondary outcomes included maternal and umbilical cord blood chemistries and maternal and neonatal outcomes, including rates of preeclampsia and preterm delivery, gestational age at delivery, and birthweight.
Results: Of note, 10 women assigned to receive pravastatin and 10 assigned to receive the placebo completed the trial. No significant differences were observed between the 2 groups in the rates of adverse or serious adverse events, congenital anomalies, or maternal and umbilical cord blood chemistries. Headache followed by heartburn and musculoskeletal pain were the most common side effects. We report the pravastatin pharmacokinetic parameters including pravastatin area under the curve (total drug exposure over a dosing interval), apparent oral clearance, half-life, and others during pregnancy and compare it with those values measured during the postpartum period. In the majority of the umbilical cord and maternal samples at the time of delivery, pravastatin concentrations were below the limit of quantification of the assay. The pregnancy and neonatal outcomes were more favorable in the pravastatin group. All newborns passed their brainstem auditory evoked response potential or similar hearing screening tests. The average maximum concentration and area under the curve values were more than 2-fold higher following a daily 20 mg dose compared with a 10 mg daily pravastatin dose, but the apparent oral clearance, half-life, and time to reach maximum concentration were similar, which is consistent with the previously reported linear, dose-independent pharmacokinetics of pravastatin in nonpregnant subjects.
Conclusion: This study confirmed the overall safety and favorable pregnancy outcomes for pravastatin in women at high risk for preeclampsia. This favorable risk-benefit analysis justifies a larger clinical trial to evaluate the efficacy of pravastatin for the prevention of preeclampsia. Until then, pravastatin use during pregnancy remains investigational.
Trial registration: ClinicalTrials.gov NCT01717586.
Keywords: angiogenic biomarkers; high-risk pregnancy; investigational new drug; maternal and neonatal morbidity; myopathy; pharmacokinetics; pilot randomized trial; pravastatin; preeclampsia; safety.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest
Figures
Similar articles
-
Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial.Am J Obstet Gynecol. 2016 Jun;214(6):720.e1-720.e17. doi: 10.1016/j.ajog.2015.12.038. Epub 2015 Dec 23. Am J Obstet Gynecol. 2016. PMID: 26723196 Free PMC article. Clinical Trial.
-
Pravastatin Versus Placebo in Pregnancies at High Risk of Term Preeclampsia.Circulation. 2021 Aug 31;144(9):670-679. doi: 10.1161/CIRCULATIONAHA.121.053963. Epub 2021 Jun 24. Circulation. 2021. PMID: 34162218 Clinical Trial.
-
INOVASIA Study: A Multicenter Randomized Clinical Trial of Pravastatin to Prevent Preeclampsia in High-Risk Patients.Am J Perinatol. 2024 Jul;41(9):1203-1211. doi: 10.1055/a-1798-1925. Epub 2022 Mar 15. Am J Perinatol. 2024. PMID: 35292944 Clinical Trial.
-
Pravastatin to treat and prevent preeclampsia. Preclinical and clinical studies.J Reprod Immunol. 2017 Nov;124:15-20. doi: 10.1016/j.jri.2017.09.009. Epub 2017 Sep 29. J Reprod Immunol. 2017. PMID: 29028516 Review.
-
Does low-dose aspirin initiated before 11 weeks' gestation reduce the rate of preeclampsia?Am J Obstet Gynecol. 2020 May;222(5):437-450. doi: 10.1016/j.ajog.2019.08.047. Epub 2019 Sep 5. Am J Obstet Gynecol. 2020. PMID: 31494125
Cited by
-
Pregnancy Complications Can Foreshadow Future Disease-Long-Term Outcomes of a Complicated Pregnancy.Medicina (Kaunas). 2021 Dec 1;57(12):1320. doi: 10.3390/medicina57121320. Medicina (Kaunas). 2021. PMID: 34946265 Free PMC article. Review.
-
One-third of patients with eclampsia at term do not have an abnormal angiogenic profile.J Perinat Med. 2022 Dec 27;51(5):652-663. doi: 10.1515/jpm-2022-0474. Print 2023 Jun 27. J Perinat Med. 2022. PMID: 36567427 Free PMC article.
-
Kidney health outcomes of hypertensive disorders of pregnancy.Nat Rev Nephrol. 2025 Jul 18. doi: 10.1038/s41581-025-00977-8. Online ahead of print. Nat Rev Nephrol. 2025. PMID: 40681847 Review.
-
The Association between Statin Use and Reduced Migraine Likelihood: A Comprehensive Analysis of Migraine Subtypes and Statin Types in a Nationwide Korean Cohort.Pharmaceuticals (Basel). 2024 Aug 10;17(8):1056. doi: 10.3390/ph17081056. Pharmaceuticals (Basel). 2024. PMID: 39204161 Free PMC article.
-
Update on Statin Use in Pregnancy.Am J Med. 2023 Jan;136(1):12-14. doi: 10.1016/j.amjmed.2022.08.029. Epub 2022 Sep 21. Am J Med. 2023. PMID: 36150512 Free PMC article. No abstract available.
References
-
- ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019;133(1):e1–e25. - PubMed
-
- Firoz T, Sanghvi H, Merialdi M, von Dadelszen P. Pre-eclampsia in low and middle income countries. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):537–548. - PubMed
-
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. - PubMed
-
- Staff AC, Fjeldstad HE, Fosheim IK, et al. Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am J Obstet Gynecol. 2020. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

