Altered maturation and atypical cortical processing of spoken sentences in autism spectrum disorder
- PMID: 34033856
- PMCID: PMC8500341
- DOI: 10.1016/j.pneurobio.2021.102077
Altered maturation and atypical cortical processing of spoken sentences in autism spectrum disorder
Abstract
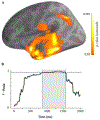
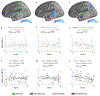
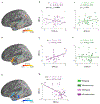
Autism spectrum disorder (ASD) is associated with widespread receptive language impairments, yet the neural mechanisms underlying these deficits are poorly understood. Neuroimaging has shown that processing of socially-relevant sounds, including speech and non-speech, is atypical in ASD. However, it is unclear how the presence of lexical-semantic meaning affects speech processing in ASD. Here, we recorded magnetoencephalography data from individuals with ASD (N = 22, ages 7-17, 4 females) and typically developing (TD) peers (N = 30, ages 7-17, 5 females) during unattended listening to meaningful auditory speech sentences and meaningless jabberwocky sentences. After adjusting for age, ASD individuals showed stronger responses to meaningless jabberwocky sentences than to meaningful speech sentences in the same left temporal and parietal language regions where TD individuals exhibited stronger responses to meaningful speech. Maturational trajectories of meaningful speech responses were atypical in temporal, but not parietal, regions in ASD. Temporal responses were associated with ASD severity, while parietal responses were associated with aberrant involuntary attentional shifting in ASD. Our findings suggest a receptive speech processing dysfunction in ASD, wherein unattended meaningful speech elicits abnormal engagement of the language system, while unattended meaningless speech, filtered out in TD individuals, engages the language system through involuntary attention capture.
Keywords: Auditory; Autism; Event-related fields; Language; Magnetoencephalography; Speech.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures


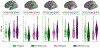
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

