Multiple parietal pathways are associated with rTMS-induced hippocampal network enhancement and episodic memory changes
- PMID: 34033914
- PMCID: PMC8926059
- DOI: 10.1016/j.neuroimage.2021.118199
Multiple parietal pathways are associated with rTMS-induced hippocampal network enhancement and episodic memory changes
Abstract
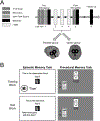
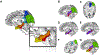
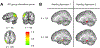
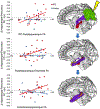
Repetitive transcranial magnetic stimulation (rTMS) of the inferior parietal cortex (IPC) increases resting-state functional connectivity (rsFC) of the hippocampus with the precuneus and other posterior cortical areas and causes proportional improvement of episodic memory. The anatomical pathway(s) responsible for the propagation of these effects from the IPC is unknown and may not be direct. In order to assess the relative contributions of candidate pathways from the IPC to the MTL via the parahippocampal cortex and precuneus, to the effects of rTMS on rsFC and memory improvement, we used diffusion tensor imaging to measure the extent to which individual differences in fractional anisotropy (FA) in these pathways accounted for individual differences in response. FA in the IPC-parahippocampal pathway and several MTL pathways predicted changes in rsFC. FA in both parahippocampal and hippocampal pathways was related to changes in episodic, but not procedural, memory. These results implicate pathways to the MTL in the enhancing effect of parietal rTMS on hippocampal rsFC and memory.
Keywords: DWI; Episodic memory; Hippocampus; Resting-state functional connectivity; rTMS.
Published by Elsevier Inc.
Figures

Similar articles
-
rTMS modulates precuneus-hippocampal subregion circuit in patients with subjective cognitive decline.Aging (Albany NY). 2020 Nov 30;13(1):1314-1331. doi: 10.18632/aging.202313. Epub 2020 Nov 30. Aging (Albany NY). 2020. PMID: 33260151 Free PMC article. Clinical Trial.
-
Frequency-specific noninvasive modulation of memory retrieval and its relationship with hippocampal network connectivity.Hippocampus. 2019 Jul;29(7):595-609. doi: 10.1002/hipo.23054. Epub 2018 Dec 11. Hippocampus. 2019. PMID: 30447076 Free PMC article.
-
Structural and functional connectivity of the precuneus and thalamus to the default mode network.Hum Brain Mapp. 2017 Feb;38(2):938-956. doi: 10.1002/hbm.23429. Epub 2016 Oct 14. Hum Brain Mapp. 2017. PMID: 27739612 Free PMC article.
-
Item, context and relational episodic encoding in humans.Curr Opin Neurobiol. 2006 Dec;16(6):693-700. doi: 10.1016/j.conb.2006.10.012. Epub 2006 Nov 9. Curr Opin Neurobiol. 2006. PMID: 17097284 Review.
-
Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: A systematic review.Neuroimage. 2020 May 1;211:116596. doi: 10.1016/j.neuroimage.2020.116596. Epub 2020 Jan 31. Neuroimage. 2020. PMID: 32014552 Free PMC article.
Cited by
-
Reproducing the effect of hippocampal network-targeted transcranial magnetic stimulation on episodic memory.Behav Brain Res. 2022 Feb 15;419:113707. doi: 10.1016/j.bbr.2021.113707. Epub 2021 Dec 7. Behav Brain Res. 2022. PMID: 34890597 Free PMC article.
-
Effectiveness of Personalized Hippocampal Network-Targeted Stimulation in Alzheimer Disease: A Randomized Clinical Trial.JAMA Netw Open. 2024 May 1;7(5):e249220. doi: 10.1001/jamanetworkopen.2024.9220. JAMA Netw Open. 2024. PMID: 38709534 Free PMC article. Clinical Trial.
-
A direct test of competitive versus cooperative episodic-procedural network dynamics in human memory.Cereb Cortex. 2022 Oct 20;32(21):4715-4732. doi: 10.1093/cercor/bhab512. Cereb Cortex. 2022. PMID: 35106536 Free PMC article.
-
Deep Brain Stimulation Therapy for Drug-Resistant Epilepsy: Present and Future Perspectives.J Epilepsy Res. 2025 Jun 10;15(1):33-41. doi: 10.14581/jer.25004. eCollection 2025 Jun. J Epilepsy Res. 2025. PMID: 40568056 Free PMC article. Review.
-
Efficacy of Cortical-Hippocampal Target Intermittent Theta Burst Stimulation (iTBS) on Associative Memory of Schizophrenia: A Double-Blind, Randomized Sham-Controlled Trial.Neuropsychiatr Dis Treat. 2024 Oct 10;20:1941-1955. doi: 10.2147/NDT.S468219. eCollection 2024. Neuropsychiatr Dis Treat. 2024. PMID: 39411184 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

