Vascular biology of uterine fibroids: connecting fibroids and vascular disorders
- PMID: 34034234
- PMCID: PMC8320308
- DOI: 10.1530/REP-21-0087
Vascular biology of uterine fibroids: connecting fibroids and vascular disorders
Abstract
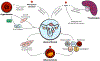
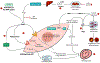
Fibroids are benign tumors caused by the proliferation of myometrial smooth muscle cells in the uterus that can lead to symptoms such as abdominal pain, constipation, urinary retention, and infertility. While traditionally thought of as a disease process intrinsic to the uterus, accumulating evidence suggests that fibroid growth may be linked with the systemic vasculature system, although cell-intrinsic factors are certainly of principal importance in their inception. Fibroids are associated with essential hypertension and preeclampsia, as well as atherosclerosis, for reasons that are becoming increasingly elucidated. Factors such as the renin-angiotensin-aldosterone system, estrogen, and endothelial dysfunction all likely play a role in fibroid pathogenesis. In this review, we lay out a framework for reconceptualizing fibroids as a systemic vascular disorder, and discuss how pharmaceutical agents and other interventions targeting the vasculature may aid in the novel treatment of fibroids.
Conflict of interest statement
Conflict of interest statement: The authors have no conflicts of interest to declare.
Figures
References
-
- ABDUL GHAFFAR NA, ISMAIL MP, NIK MAHMOOD NM, DAUD K & ABU DZARR GA 2008. Huge uterine fibroid in a postmenopausal woman associated with polycythaemia: a case report. Maturitas, 60, 177–9. - PubMed
-
- AGRAWAL R, PRELEVIC G, CONWAY GS, PAYNE NN, GINSBURG J & JACOBS HS 2000. Serum vascular endothelial growth factor concentrations in postmenopausal women: the effect of hormone replacement therapy. Fertil Steril, 73, 56–60. - PubMed
-
- AKSOY Y, SIVRI N, KARAOZ B, SAYIN C & YETKIN E 2014. Carotid intima-media thickness: a new marker of patients with uterine leiomyoma. Eur J Obstet Gynecol Reprod Biol, 175, 54–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

