Long non-coding RNAs in Epstein-Barr virus-related cancer
- PMID: 34034760
- PMCID: PMC8144696
- DOI: 10.1186/s12935-021-01986-w
Long non-coding RNAs in Epstein-Barr virus-related cancer
Abstract
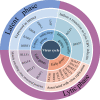
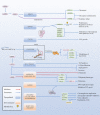
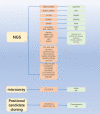
Epstein Barr-virus (EBV) is related to several cancers. Long non-coding RNAs (lncRNAs) act by regulating target genes and are involved in tumourigenesis. However, the role of lncRNAs in EBV-associated cancers is rarely reported. Understanding the role and mechanism of lncRNAs in EBV-associated cancers may contribute to diagnosis, prognosis and clinical therapy in the future. EBV encodes not only miRNAs, but also BART lncRNAs during latency and the BHLF1 lncRNA during both the latent and lytic phases. These lncRNAs can be targeted regulate inflammation, invasion, and migration and thus tumourigenesis. The products of EBV also directly and indirectly regulate host lncRNAs, including LINC00312, NORAD CYTOR, SHNG8, SHNG5, MINCR, lncRNA-BC200, LINC00672, MALATI1, LINC00982, LINC02067, IGFBP7-AS1, LOC100505716, LOC100128494, NAG7 and RP4-794H19.1, to facilitate tumourigenesis using different mechanisms. Additionally, lncRNAs have been previously validated to interact with microRNAs (miRNAs), and lncRNAs and miRNAs mutually suppress each other. The EBV-miR-BART6-3p/LOC553103/STMN1 axis inhibits EBV-associated tumour cell proliferation. Additionally, H. pylori-EBV co-infection promotes inflammatory lesions and results in EMT. HPV-EBV co-infection inhibits the transition from latency to lytic replication. KSHV-EBV co-infection aggravates tumourigenesis in huNSG mice. COVID-19-EBV co-infection may activate the immune system to destroy a tumour, although this situation is rare and the mechanism requires further confirmation. Hopefully, this information will shed some light on tumour therapy strategies tumourigenesis. Additionally, this strategy benefits for infected patients by preventing latency to lytic replication. Understanding the role and expression of lnRNAs in these two phases of EBV is critical to control the transition from latency to the lytic replication phase. This review presents differential expressed lncRNAs in EBV-associated cancers and provides resources to aid in developing superior strategies for clinical therapy.
Keywords: EBV latent infection; EBV lytic infection; Epstein–Barr virus; Long non-coding RNAs (lncRNAs); Tumourigenesis.
Conflict of interest statement
All the authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Epstein-Barr virus-encoded miR-BART6-3p inhibits cancer cell proliferation through the LOC553103-STMN1 axis.FASEB J. 2020 Jun;34(6):8012-8027. doi: 10.1096/fj.202000039RR. Epub 2020 Apr 18. FASEB J. 2020. PMID: 32306460
-
Epstein-Barr virus-encoded miR-BART6-3p inhibits cancer cell metastasis and invasion by targeting long non-coding RNA LOC553103.Cell Death Dis. 2016 Sep 1;7(9):e2353. doi: 10.1038/cddis.2016.253. Cell Death Dis. 2016. PMID: 27584792 Free PMC article.
-
The BHLF1 Locus of Epstein-Barr Virus Contributes to Viral Latency and B-Cell Immortalization.J Virol. 2020 Aug 17;94(17):e01215-20. doi: 10.1128/JVI.01215-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581094 Free PMC article.
-
Long noncoding RNAs involvement in Epstein-Barr virus infection and tumorigenesis.Virol J. 2020 Apr 9;17(1):51. doi: 10.1186/s12985-020-01308-y. Virol J. 2020. PMID: 32272952 Free PMC article. Review.
-
Role of microRNAs and Exosomes in Helicobacter pylori and Epstein-Barr Virus Associated Gastric Cancers.Front Microbiol. 2018 Apr 5;9:636. doi: 10.3389/fmicb.2018.00636. eCollection 2018. Front Microbiol. 2018. PMID: 29675003 Free PMC article. Review.
Cited by
-
Epstein‑Barr virus as a promoter of tumorigenesis in the tumor microenvironment of breast cancer (Review).Int J Mol Med. 2023 Aug;52(2):72. doi: 10.3892/ijmm.2023.5275. Epub 2023 Jul 7. Int J Mol Med. 2023. PMID: 37417334 Free PMC article. Review.
-
A comprehensive prognostic and immune analysis of enhancer RNA identifies IGFBP7-AS1 as a novel prognostic biomarker in Uterine Corpus Endometrial Carcinoma.Biol Proced Online. 2022 Jul 15;24(1):9. doi: 10.1186/s12575-022-00172-0. Biol Proced Online. 2022. PMID: 35836132 Free PMC article.
-
Mono a Mano: ZBP1's Love-Hate Relationship with the Kissing Virus.Int J Mol Sci. 2022 Mar 12;23(6):3079. doi: 10.3390/ijms23063079. Int J Mol Sci. 2022. PMID: 35328502 Free PMC article. Review.
-
SNHG8 Promotes the Progression of Epstein-Barr Virus-Associated Gastric Cancer via Sponging miR-512-5p and Targeting TRIM28.Front Oncol. 2021 Oct 15;11:734694. doi: 10.3389/fonc.2021.734694. eCollection 2021. Front Oncol. 2021. PMID: 34722282 Free PMC article.
-
The viral etiology of EBV-associated gastric cancers contributes to their unique pathology, clinical outcomes, treatment responses and immune landscape.Front Immunol. 2024 Mar 26;15:1358511. doi: 10.3389/fimmu.2024.1358511. eCollection 2024. Front Immunol. 2024. PMID: 38596668 Free PMC article. Review.
References
-
- Hewitt LC, Inam IZ, Saito Y, Yoshikawa T, Quaas A, Hoelscher A, Bollschweiler E, Fazzi GE, Melotte V, Langley RE, et al. Epstein-Barr virus and mismatch repair deficiency status differ between oesophageal and gastric cancer: a large multi-centre study. Eur J Cancer. 2018;94:104–114. doi: 10.1016/j.ejca.2018.02.014. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

