Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease
- PMID: 34035003
- PMCID: PMC8219484
- DOI: 10.1136/annrheumdis-2021-220597
Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease
Abstract
Objective: To investigate the humoral and cellular immune response to messenger RNA (mRNA) COVID-19 vaccines in patients with immune-mediated inflammatory diseases (IMIDs) on immunomodulatory treatment.
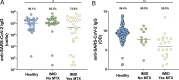
Methods: Established patients at New York University Langone Health with IMID (n=51) receiving the BNT162b2 mRNA vaccination were assessed at baseline and after second immunisation. Healthy subjects served as controls (n=26). IgG antibody responses to the spike protein were analysed for humoral response. Cellular immune response to SARS-CoV-2 was further analysed using high-parameter spectral flow cytometry. A second independent, validation cohort of controls (n=182) and patients with IMID (n=31) from Erlangen, Germany, were also analysed for humoral immune response.
Results: Although healthy subjects (n=208) and patients with IMID on biologic treatments (mostly on tumour necrosis factor blockers, n=37) demonstrate robust antibody responses (over 90%), those patients with IMID on background methotrexate (n=45) achieve an adequate response in only 62.2% of cases. Similarly, patients with IMID on methotrexate do not demonstrate an increase in CD8+ T-cell activation after vaccination.
Conclusions: In two independent cohorts of patients with IMID, methotrexate, a widely used immunomodulator for the treatment of several IMIDs, adversely affected humoral and cellular immune response to COVID-19 mRNA vaccines. Although precise cut-offs for immunogenicity that correlate with vaccine efficacy are yet to be established, our findings suggest that different strategies may need to be explored in patients with IMID taking methotrexate to increase the chances of immunisation efficacy against SARS-CoV-2 as has been demonstrated for augmenting immunogenicity to other viral vaccines.
Keywords: Covid-19; arthritis; methotrexate; psoriatic; rheumatoid; vaccination.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JUS declares that he has served as a consultant for Janssen, Novartis, Pfizer, Sanofi, UCB and Abbvie and has received funding for investigator-initiated studies from Novartis, Sanofi and Janssen. GS has served as a consultant for Abbvie, BMS, Eli Lilly, Gilead, GSK Novartis, Janssen and Roche and has received funding for investigator-initiated studies from BMS, Eli Lilly, GSK, Novartis and UCB. MM declares grants from Eli Lilly, Pfizer and Sanofi and personal fees from Meissa Vaccines. PI has received consulting fees from GSK. RHH has received consulting from Janssen. SA reports grant support from Johnson and Johnson. GS declares consulting fees from AbbVie.
Figures

Update of
-
Methotrexate Hampers Immunogenicity to BNT162b2 mRNA COVID-19 Vaccine in Immune-Mediated Inflammatory Disease.medRxiv [Preprint]. 2021 May 12:2021.05.11.21256917. doi: 10.1101/2021.05.11.21256917. medRxiv. 2021. Update in: Ann Rheum Dis. 2021 Oct;80(10):1339-1344. doi: 10.1136/annrheumdis-2021-220597. PMID: 34013285 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

