Intranasal Administration of Oxytocin Attenuates Social Recognition Deficits and Increases Prefrontal Cortex Inhibitory Postsynaptic Currents following Traumatic Brain Injury
- PMID: 34035071
- PMCID: PMC8205495
- DOI: 10.1523/ENEURO.0061-21.2021
Intranasal Administration of Oxytocin Attenuates Social Recognition Deficits and Increases Prefrontal Cortex Inhibitory Postsynaptic Currents following Traumatic Brain Injury
Abstract
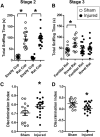
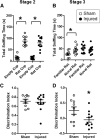
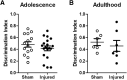
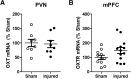
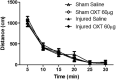
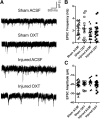
Pediatric traumatic brain injury (TBI) results in heightened risk for social deficits that can emerge during adolescence and adulthood. A moderate TBI in male and female rats on postnatal day 11 (equivalent to children below the age of 4) resulted in impairments in social novelty recognition, defined as the preference for interacting with a novel rat compared with a familiar rat, but not sociability, defined as the preference for interacting with a rat compared with an object in the three-chamber test when tested at four weeks (adolescence) and eight weeks (adulthood) postinjury. The deficits in social recognition were not accompanied by deficits in novel object recognition memory and were associated with a decrease in the frequency of spontaneous inhibitory postsynaptic currents (IPSCs) recorded from pyramidal neurons within Layer II/III of the medial prefrontal cortex (mPFC). Whereas TBI did not affect the expression of oxytocin (OXT) or the OXT receptor (OXTR) mRNAs in the hypothalamus and mPFC, respectively, intranasal administration of OXT before behavioral testing was found to reduce impairments in social novelty recognition and increase IPSC frequency in the mPFC in brain-injured animals. These results suggest that TBI-induced deficits in social behavior may be linked to increased excitability of neurons in the mPFC and suggests that the regulation of GABAergic neurotransmission in this region as a potential mechanism underlying these deficits.
Keywords: GABAergic neurotransmission; excitability; intranasal administration; oxytocin; pediatric TBI; social behavior.
Copyright © 2021 Runyan et al.
Figures


Similar articles
-
Progesterone treatment following traumatic brain injury in the 11-day-old rat attenuates cognitive deficits and neuronal hyperexcitability in adolescence.Exp Neurol. 2020 Aug;330:113329. doi: 10.1016/j.expneurol.2020.113329. Epub 2020 Apr 23. Exp Neurol. 2020. PMID: 32335121 Free PMC article.
-
Effects of oxytocin on prosocial behavior and the associated profiles of oxytocinergic and corticotropin-releasing hormone receptors in a rodent model of posttraumatic stress disorder.J Biomed Sci. 2019 Mar 21;26(1):26. doi: 10.1186/s12929-019-0514-0. J Biomed Sci. 2019. PMID: 30898126 Free PMC article.
-
Conditional Deletion of Hippocampal CA2/CA3a Oxytocin Receptors Impairs the Persistence of Long-Term Social Recognition Memory in Mice.J Neurosci. 2018 Jan 31;38(5):1218-1231. doi: 10.1523/JNEUROSCI.1896-17.2017. Epub 2017 Dec 26. J Neurosci. 2018. PMID: 29279308 Free PMC article.
-
The role of oxytocin receptor gene (OXTR) DNA methylation (DNAm) in human social and emotional functioning: a systematic narrative review.BMC Psychiatry. 2018 May 29;18(1):154. doi: 10.1186/s12888-018-1740-9. BMC Psychiatry. 2018. PMID: 29843655 Free PMC article.
-
The Oxytocin Receptor: From Intracellular Signaling to Behavior.Physiol Rev. 2018 Jul 1;98(3):1805-1908. doi: 10.1152/physrev.00031.2017. Physiol Rev. 2018. PMID: 29897293 Review.
Cited by
-
A Pro-social Pill? The Potential of Pharmacological Treatments to Improve Social Outcomes After Pediatric Traumatic Brain Injury.Front Neurol. 2021 Aug 19;12:714253. doi: 10.3389/fneur.2021.714253. eCollection 2021. Front Neurol. 2021. PMID: 34489853 Free PMC article. Review.
-
Effects of a 33-ion sequential beam galactic cosmic ray analog on male mouse behavior and evaluation of CDDO-EA as a radiation countermeasure.Behav Brain Res. 2022 Feb 15;419:113677. doi: 10.1016/j.bbr.2021.113677. Epub 2021 Nov 21. Behav Brain Res. 2022. PMID: 34818568 Free PMC article.
-
Editorial: Long-term consequences of pediatric traumatic brain injury: Improved understanding to help young patients survive and thrive.Front Neurol. 2022 Sep 9;13:1011998. doi: 10.3389/fneur.2022.1011998. eCollection 2022. Front Neurol. 2022. PMID: 36158951 Free PMC article. No abstract available.
-
Importance of Control Groups for Evaluating Long-Term Behavioral and Cognitive Outcomes of Controlled Cortical Impact in Immature Rats.J Neurotrauma. 2023 Jun;40(11-12):1197-1215. doi: 10.1089/neu.2021.0376. Epub 2023 Mar 1. J Neurotrauma. 2023. PMID: 36416234 Free PMC article.
-
Moderate Intensity of Treadmill Exercise Rescues TBI-Induced Ferroptosis, Neurodegeneration, and Cognitive Impairments via Suppressing STING Pathway.Mol Neurobiol. 2023 Sep;60(9):4872-4896. doi: 10.1007/s12035-023-03379-8. Epub 2023 May 16. Mol Neurobiol. 2023. PMID: 37193866 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
