Measuring the impact of COVID-19 on the quality of life of the survivors, partners and family members: a cross-sectional international online survey
- PMID: 34035105
- PMCID: PMC8154981
- DOI: 10.1136/bmjopen-2020-047680
Measuring the impact of COVID-19 on the quality of life of the survivors, partners and family members: a cross-sectional international online survey
Abstract
Objective: This study aimed to measure the impact of COVID-19 on the quality of life (QoL) of survivors and their partners and family members.
Design and setting: A prospective cross-sectional global online survey using social media.
Participants: Patients with COVID-19 and partners or family members (age ≥18 years).
Intervention: Online survey from June to August 2020.
Main outcome measure: The EuroQol group five dimensions three level (EQ-5D-3L) to measure the QoL of survivors of COVID-19, and the Family Reported Outcome Measure (FROM-16) to assess the impact on their partner/family member's QoL.
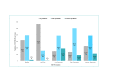
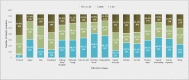
Results: The survey was completed by 735 COVID-19 survivors (mean age=48 years; females=563) at a mean of 12.8 weeks after diagnosis and by 571 partners and 164 family members (n=735; mean age=47 years; females=246) from Europe (50.6%), North America (38.5%) and rest of the world (10.9%). The EQ-5D mean score for COVID-19 survivors was 8.65 (SD=1.9, median=9; range=6-14). 81.1% (596/735) reported pain and discomfort, 79.5% (584/735) problems with usual activities, 68.7% (505/735) anxiety and depression and 56.2% (413/735) problems with mobility. Hospitalised survivors (20.1%, n=148) and survivors with existing health conditions (30.9%, n=227) reported significantly more problems with mobility and usual activities (p<0.05), with hospitalised also experiencing more impact on self-care (p≤0.001). Among 735 partners and family members, the mean FROM-16 score (maximum score=highest impact =32) was 15 (median=15, range=0-32). 93.6% (688/735) reported being worried, 81.7% (601/735) frustrated, 78.4% (676/735) sad, 83.3% (612/735) reported impact on their family activities, 68.9% (507/735) on sleep and 68.1% (500/735) on their sex life.
Conclusion: COVID-19 survivors reported a major persisting impact on their physical and psychosocial health. The lives of their partners and other family members were also severely affected. There is a need for a holistic support system sensitive to the needs of COVID-19 survivors and their family members who experience a major 'secondary burden'.
Keywords: COVID-19; infection control; public health.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RS and SJN declared no competing interest, AYF is joint copyright holder of FROM-16 and of other quality of life measures. AYF reports personal fees from Novartis. SMS is joint copyright holder of FROM-16 and of other quality of life measures. JRI reports personal fees from UCB Pharma, personal fees from Novartis, personal fees from Boehringer Ingelheim, personal fees from Kymera Therapeutics, personal fees from Viela Bio, personal fees from UpToDate, personal fees from Editor of British Journal of Dermatology, outside the submitted work; in addition, JRI is co-copyright holder of the Hidradenitis Suppurativa Quality of Life (HiSQOL) score. FA reports grants and personal fees from Janssen, personal fees from AbbVie, personal fees from Lilly Pharmaceuticals, personal fees from L'Oreal, personal fees from LEO Pharmaceuticals, personal fees from UCB, outside the submitted work.
Figures
References
-
- Jisc . Online surveys. Jisc, Bristol, UK, 2020. Available: https://www.onlinesurveys.ac.uk/
-
- EQ-5D-3L . User guide, 2018. Available: https://euroqol.org/publications/user-guides
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical