Understanding T cell phenotype for the design of effective chimeric antigen receptor T cell therapies
- PMID: 34035114
- PMCID: PMC8154965
- DOI: 10.1136/jitc-2021-002555
Understanding T cell phenotype for the design of effective chimeric antigen receptor T cell therapies
Abstract
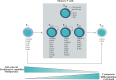
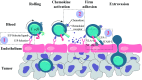
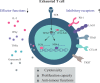
Rapid advances in immunotherapy have identified adoptive cell transfer as one of the most promising approaches for the treatment of cancers. Large numbers of cancer reactive T lymphocytes can be generated ex vivo from patient blood by genetic modification to express chimeric antigen receptors (CAR) specific for tumor-associated antigens. CAR T cells can respond strongly against cancer cells, and adoptive transferred CAR T cells can induce dramatic responses against certain types of cancers. The ability of T cells to respond against disease depends on their ability to localize to sites, persist and exert functions, often in an immunosuppressive microenvironment, and these abilities are reflected in their phenotypes. There is currently intense interest in generating CAR T cells possessing the ideal phenotypes to confer optimal antitumor activity. In this article, we review T cell phenotypes for trafficking, persistence and function, and discuss how culture conditions and genetic makeups can be manipulated to achieve the ideal phenotypes for antitumor activities.
Keywords: chimeric antigen receptor; exhaustion; memory; persistence; self-renewal; trafficking.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
CAR T-cell therapy for solid tumours.Lancet Oncol. 2021 Jul;22(7):893. doi: 10.1016/S1470-2045(21)00353-3. Lancet Oncol. 2021. PMID: 34197740 No abstract available.
-
Single-cell multiomics dissection of basal and antigen-specific activation states of CD19-targeted CAR T cells.J Immunother Cancer. 2021 May;9(5):e002328. doi: 10.1136/jitc-2020-002328. J Immunother Cancer. 2021. PMID: 34006631 Free PMC article.
-
Genetic Modification of Cytokine Signaling to Enhance Efficacy of CAR T Cell Therapy in Solid Tumors.Front Immunol. 2021 Oct 14;12:738456. doi: 10.3389/fimmu.2021.738456. eCollection 2021. Front Immunol. 2021. PMID: 34721401 Free PMC article. Review.
-
Engineering chimeric antigen receptor-T cells for cancer treatment.Mol Cancer. 2018 Feb 15;17(1):32. doi: 10.1186/s12943-018-0814-0. Mol Cancer. 2018. PMID: 29448937 Free PMC article. Review.
-
Immune Cell Hacking: Challenges and Clinical Approaches to Create Smarter Generations of Chimeric Antigen Receptor T Cells.Front Immunol. 2018 Jul 31;9:1717. doi: 10.3389/fimmu.2018.01717. eCollection 2018. Front Immunol. 2018. PMID: 30108584 Free PMC article. Review.
Cited by
-
Product Attributes of CAR T-cell Therapy Differentially Associate with Efficacy and Toxicity in Second-line Large B-cell Lymphoma (ZUMA-7).Blood Cancer Discov. 2024 Jan 8;5(1):21-33. doi: 10.1158/2643-3230.BCD-23-0112. Blood Cancer Discov. 2024. PMID: 37983485 Free PMC article.
-
Droplet Microfluidics Enables Tracing of Target Cells at the Single-Cell Transcriptome Resolution.Bioengineering (Basel). 2022 Nov 10;9(11):674. doi: 10.3390/bioengineering9110674. Bioengineering (Basel). 2022. PMID: 36354585 Free PMC article.
-
Cellular kinetics: A clinical and computational review of CAR-T cell pharmacology.Adv Drug Deliv Rev. 2022 Sep;188:114421. doi: 10.1016/j.addr.2022.114421. Epub 2022 Jul 6. Adv Drug Deliv Rev. 2022. PMID: 35809868 Free PMC article. Review.
-
Novel therapeutic agents in clinical trials: emerging approaches in cancer therapy.Discov Oncol. 2024 Aug 11;15(1):342. doi: 10.1007/s12672-024-01195-7. Discov Oncol. 2024. PMID: 39127974 Free PMC article. Review.
-
Enhancing co-stimulation of CAR T cells to improve treatment outcomes in solid cancers.Immunother Adv. 2021 Jul 31;1(1):ltab016. doi: 10.1093/immadv/ltab016. eCollection 2021 Jan. Immunother Adv. 2021. PMID: 35919743 Free PMC article. Review.
References
-
- Slaney CY, Wang P, Darcy PK, et al. . CARs versus BiTEs: a comparison between T Cell-Redirection strategies for cancer treatment. Cancer Discov 2018;8:924-934. 10.1158/2159-8290.CD-18-0297 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
