SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees
- PMID: 34035118
- PMCID: PMC9268159
- DOI: 10.1126/sciimmunol.abj1750
SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees
Abstract
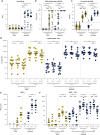
The emergence of SARS-CoV-2 variants harboring mutations in the spike (S) protein has raised concern about potential immune escape. Here, we studied humoral and cellular immune responses to wild type SARS-CoV-2 and the B.1.1.7 and B.1.351 variants of concern in a cohort of 121 BNT162b2 mRNA-vaccinated health care workers (HCW). Twenty-three HCW recovered from mild COVID-19 disease and exhibited a recall response with high levels of SARS-CoV-2-specific functional antibodies and virus-specific T cells after a single vaccination. Specific immune responses were also detected in seronegative HCW after one vaccination, but a second dose was required to reach high levels of functional antibodies and cellular immune responses in all individuals. Vaccination-induced antibodies cross-neutralized the variants B.1.1.7 and B.1.351, but the neutralizing capacity and Fc-mediated functionality against B.1.351 was consistently 2- to 4-fold lower than to the homologous virus. In addition, peripheral blood mononuclear cells were stimulated with peptide pools spanning the mutated S regions of B.1.1.7 and B.1.351 to detect cross-reactivity of SARS-CoV-2-specific T cells with variants. Importantly, we observed no differences in CD4+ T-cell activation in response to variant antigens, indicating that the B.1.1.7 and B.1.351 S proteins do not escape T-cell-mediated immunity elicited by the wild type S protein. In conclusion, this study shows that some variants can partially escape humoral immunity induced by SARS-CoV-2 infection or BNT162b2 vaccination, but S-specific CD4+ T-cell activation is not affected by the mutations in the B.1.1.7 and B.1.351 variants.
Copyright © 2021, American Association for the Advancement of Science.
Figures


References
-
- Hale T., Angrist N., Goldszmidt R., Kira B., Petherick A., Phillips T., Webster S., Cameron-Blake E., Hallas L., Majumdar S., Tatlow H., A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 5, 529–538 (2021). - PubMed
-
- Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F., Baysal E., Mangold M., Henze L., Lauster R., Mall M. A., Beyer K., Rohmel J., Voigt S., Schmitz J., Miltenyi S., Demuth I., Muller M. A., Hocke A., Witzenrath M., Suttorp N., Kern F., Reimer U., Wenschuh H., Drosten C., Corman V. M., Giesecke-Thiel C., Sander L. E., Thiel A., SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 587, 270–274 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

