Phosphorylation of Pal2 by the protein kinases Kin1 and Kin2 modulates HAC1 mRNA splicing in the unfolded protein response in yeast
- PMID: 34035143
- PMCID: PMC8285061
- DOI: 10.1126/scisignal.aaz4401
Phosphorylation of Pal2 by the protein kinases Kin1 and Kin2 modulates HAC1 mRNA splicing in the unfolded protein response in yeast
Abstract
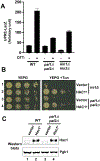
During cellular stress in the budding yeast Saccharomyces cerevisiae, an endoplasmic reticulum (ER)-resident dual kinase and RNase Ire1 splices an intron from HAC1 mRNA in the cytosol, thereby releasing its translational block. Hac1 protein then activates an adaptive cellular stress response called the unfolded protein response (UPR) that maintains ER homeostasis. The polarity-inducing protein kinases Kin1 and Kin2 contribute to HAC1 mRNA processing. Here, we showed that an RNA-protein complex that included the endocytic proteins Pal1 and Pal2 mediated HAC1 mRNA splicing downstream of Kin1 and Kin2. We found that Pal1 and Pal2 bound to the 3' untranslated region (3'UTR) of HAC1 mRNA, and a yeast strain lacking both Pal1 and Pal2 was deficient in HAC1 mRNA processing. We also showed that Kin1 and Kin2 directly phosphorylated Pal2, and that a nonphosphorylatable Pal2 mutant could not rescue the UPR defect in a pal1Δ pal2Δ strain. Thus, our work uncovers a Kin1/2-Pal2 signaling pathway that coordinates HAC1 mRNA processing and ER homeostasis.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
COMPETING INTERESTS:
The authors declare that they have no competing interests.
Figures






References
-
- Walter P, and Ron D (2011) The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 334, 1081–1086 - PubMed
-
- Ron D, and Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Bio 8, 519–529 - PubMed
-
- Cox JS, and Walter P (1996) A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

