The P-type ATPase transporter ATP7A promotes angiogenesis by limiting autophagic degradation of VEGFR2
- PMID: 34035268
- PMCID: PMC8149886
- DOI: 10.1038/s41467-021-23408-1
The P-type ATPase transporter ATP7A promotes angiogenesis by limiting autophagic degradation of VEGFR2
Abstract
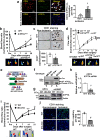
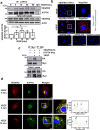
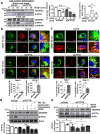
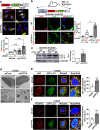
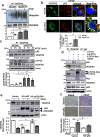
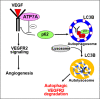
VEGFR2 (KDR/Flk1) signaling in endothelial cells (ECs) plays a central role in angiogenesis. The P-type ATPase transporter ATP7A regulates copper homeostasis, and its role in VEGFR2 signaling and angiogenesis is entirely unknown. Here, we describe the unexpected crosstalk between the Copper transporter ATP7A, autophagy, and VEGFR2 degradation. The functional significance of this Copper transporter was demonstrated by the finding that inducible EC-specific ATP7A deficient mice or ATP7A-dysfunctional ATP7Amut mice showed impaired post-ischemic neovascularization. In ECs, loss of ATP7A inhibited VEGF-induced VEGFR2 signaling and angiogenic responses, in part by promoting ligand-induced VEGFR2 protein degradation. Mechanistically, VEGF stimulated ATP7A translocation from the trans-Golgi network to the plasma membrane where it bound to VEGFR2, which prevented autophagy-mediated lysosomal VEGFR2 degradation by inhibiting autophagic cargo/adapter p62/SQSTM1 binding to ubiquitinated VEGFR2. Enhanced autophagy flux due to ATP7A dysfunction in vivo was confirmed by autophagy reporter CAG-ATP7Amut -RFP-EGFP-LC3 transgenic mice. In summary, our study uncovers a novel function of ATP7A to limit autophagy-mediated degradation of VEGFR2, thereby promoting VEGFR2 signaling and angiogenesis, which restores perfusion recovery and neovascularization. Thus, endothelial ATP7A is identified as a potential therapeutic target for treatment of ischemic cardiovascular diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Akt2 (Protein Kinase B Beta) Stabilizes ATP7A, a Copper Transporter for Extracellular Superoxide Dismutase, in Vascular Smooth Muscle: Novel Mechanism to Limit Endothelial Dysfunction in Type 2 Diabetes Mellitus.Arterioscler Thromb Vasc Biol. 2018 Mar;38(3):529-541. doi: 10.1161/ATVBAHA.117.309819. Epub 2018 Jan 4. Arterioscler Thromb Vasc Biol. 2018. PMID: 29301787 Free PMC article.
-
Caveolin-1 stabilizes ATP7A, a copper transporter for extracellular SOD, in vascular tissue to maintain endothelial function.Am J Physiol Cell Physiol. 2020 Nov 1;319(5):C933-C944. doi: 10.1152/ajpcell.00151.2020. Epub 2020 Sep 16. Am J Physiol Cell Physiol. 2020. PMID: 32936699 Free PMC article.
-
Endothelial SCUBE2 Interacts With VEGFR2 and Regulates VEGF-Induced Angiogenesis.Arterioscler Thromb Vasc Biol. 2017 Jan;37(1):144-155. doi: 10.1161/ATVBAHA.116.308546. Epub 2016 Nov 10. Arterioscler Thromb Vasc Biol. 2017. PMID: 27834687
-
Autophagy at the interface of endothelial cell homeostasis and vascular disease.FEBS J. 2022 Jun;289(11):2976-2991. doi: 10.1111/febs.15873. Epub 2021 May 2. FEBS J. 2022. PMID: 33934518 Review.
-
Autophagy as a Guardian of Vascular Niche Homeostasis.Int J Mol Sci. 2024 Sep 20;25(18):10097. doi: 10.3390/ijms251810097. Int J Mol Sci. 2024. PMID: 39337582 Free PMC article. Review.
Cited by
-
Copper in Gynecological Diseases.Int J Mol Sci. 2023 Dec 17;24(24):17578. doi: 10.3390/ijms242417578. Int J Mol Sci. 2023. PMID: 38139406 Free PMC article. Review.
-
Protein disulfide isomerase A1 as a novel redox sensor in VEGFR2 signaling and angiogenesis.Angiogenesis. 2023 Feb;26(1):77-96. doi: 10.1007/s10456-022-09852-7. Epub 2022 Aug 19. Angiogenesis. 2023. PMID: 35984546 Free PMC article.
-
Cell Death in Hepatocellular Carcinoma: Pathogenesis and Therapeutic Opportunities.Cancers (Basel). 2021 Dec 23;14(1):48. doi: 10.3390/cancers14010048. Cancers (Basel). 2021. PMID: 35008212 Free PMC article. Review.
-
Copper-binding protein modelling by single-cell transcriptome and Bulk transcriptome to predict overall survival in lung adenocarcinoma patients.J Cancer. 2024 Mar 17;15(9):2659-2677. doi: 10.7150/jca.94588. eCollection 2024. J Cancer. 2024. PMID: 38577594 Free PMC article.
-
Mammalian copper homeostasis: physiological roles and molecular mechanisms.Physiol Rev. 2025 Jan 1;105(1):441-491. doi: 10.1152/physrev.00011.2024. Epub 2024 Aug 22. Physiol Rev. 2025. PMID: 39172219 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

