Non-productive angiogenesis disassembles Aß plaque-associated blood vessels
- PMID: 34035282
- PMCID: PMC8149638
- DOI: 10.1038/s41467-021-23337-z
Non-productive angiogenesis disassembles Aß plaque-associated blood vessels
Abstract
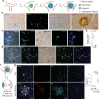
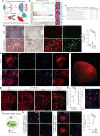
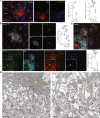
The human Alzheimer's disease (AD) brain accumulates angiogenic markers but paradoxically, the cerebral microvasculature is reduced around Aß plaques. Here we demonstrate that angiogenesis is started near Aß plaques in both AD mouse models and human AD samples. However, endothelial cells express the molecular signature of non-productive angiogenesis (NPA) and accumulate, around Aß plaques, a tip cell marker and IB4 reactive vascular anomalies with reduced NOTCH activity. Notably, NPA induction by endothelial loss of presenilin, whose mutations cause familial AD and which activity has been shown to decrease with age, produced a similar vascular phenotype in the absence of Aß pathology. We also show that Aß plaque-associated NPA locally disassembles blood vessels, leaving behind vascular scars, and that microglial phagocytosis contributes to the local loss of endothelial cells. These results define the role of NPA and microglia in local blood vessel disassembly and highlight the vascular component of presenilin loss of function in AD.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

