Immune response to SARS-CoV-2 variants of concern in vaccinated individuals
- PMID: 34035301
- PMCID: PMC8149389
- DOI: 10.1038/s41467-021-23473-6
Immune response to SARS-CoV-2 variants of concern in vaccinated individuals
Abstract
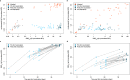
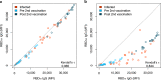
SARS-CoV-2 is evolving with mutations in the receptor binding domain (RBD) being of particular concern. It is important to know how much cross-protection is offered between strains following vaccination or infection. Here, we obtain serum and saliva samples from groups of vaccinated (Pfizer BNT-162b2), infected and uninfected individuals and characterize the antibody response to RBD mutant strains. Vaccinated individuals have a robust humoral response after the second dose and have high IgG antibody titers in the saliva. Antibody responses however show considerable differences in binding to RBD mutants of emerging variants of concern and substantial reduction in RBD binding and neutralization is observed against a patient-isolated South African variant. Taken together our data reinforce the importance of the second dose of Pfizer BNT-162b2 to acquire high levels of neutralizing antibodies and high antibody titers in saliva suggest that vaccinated individuals may have reduced transmission potential. Substantially reduced neutralization for the South African variant further highlights the importance of surveillance strategies to detect new variants and targeting these in future vaccines.
Conflict of interest statement
T.R.W., P.K., N.S.M., and U.R. are named as inventors on a patent application (EP 20 197 031.6) claiming the use of the described Nanobodies used in the NeutrobodyPlex for diagnosis and therapeutics filed by the Natural and Medical Sciences Institute. N.S.-M. was a speaker at Luminex user meetings in the past. The Natural and Medical Sciences Institute at the University of Tuebingen is involved in applied research projects as a fee for services with Luminex. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- DFG-KO 3884/5-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SO-96/Helmholtz Association
- 101003480-CORESMA/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- FKZ-3-4332.62-NMI-67/Ministerium für Wirtschaft, Arbeit und Wohnungsbau Baden-Württemberg (Ministry of Economics, Labor and Housing of Baden-Württemberg)
- FKZ-3-4332.62-NMI-68/Ministerium für Wirtschaft, Arbeit und Wohnungsbau Baden-Württemberg (Ministry of Economics, Labor and Housing of Baden-Württemberg)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

