A predictive model for vertebrate bone identification from collagen using proteomic mass spectrometry
- PMID: 34035355
- PMCID: PMC8149876
- DOI: 10.1038/s41598-021-90231-5
A predictive model for vertebrate bone identification from collagen using proteomic mass spectrometry
Abstract
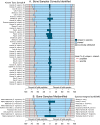
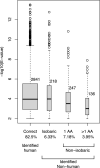
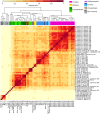
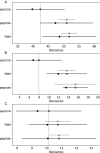
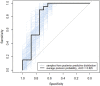
Proteogenomics is an increasingly common method for species identification as it allows for rapid and inexpensive interrogation of an unknown organism's proteome-even when the proteome is partially degraded. The proteomic method typically uses tandem mass spectrometry to survey all peptides detectable in a sample that frequently contains hundreds or thousands of proteins. Species identification is based on detection of a small numbers of species-specific peptides. Genetic analysis of proteins by mass spectrometry, however, is a developing field, and the bone proteome, typically consisting of only two proteins, pushes the limits of this technology. Nearly 20% of highly confident spectra from modern human bone samples identify non-human species when searched against a vertebrate database-as would be necessary with a fragment of unknown bone. These non-human peptides are often the result of current limitations in mass spectrometry or algorithm interpretation errors. Consequently, it is difficult to know if a "species-specific" peptide used to identify a sample is actually present in that sample. Here we evaluate the causes of peptide sequence errors and propose an unbiased, probabilistic approach to determine the likelihood that a species is correctly identified from bone without relying on species-specific peptides.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

