Kinship of conditionally immortalized cells derived from fetal bone to human bone-derived mesenchymal stroma cells
- PMID: 34035368
- PMCID: PMC8149839
- DOI: 10.1038/s41598-021-90161-2
Kinship of conditionally immortalized cells derived from fetal bone to human bone-derived mesenchymal stroma cells
Abstract
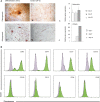
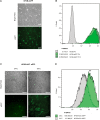
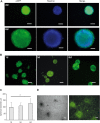
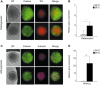
The human fetal osteoblast cell line (hFOB 1.19) has been proposed as an accessible experimental model for study of osteoblast biology relating to drug development and biomaterial engineering. For their multilineage differentiation potential, hFOB has been compared to human mesenchymal progenitor cells and used to investigate bone-metabolism in vitro. Hereby, we studied whether and to what extent the conditionally immortalized cell line hFOB 1.19 can serve as a surrogate model for bone-marrow derived mesenchymal stromal cells (bmMSC). hFOB indeed exhibit specific characteristics reminiscent of bmMSC, as colony formation, migration capacity and the propensity to grow as multicellular aggregates. After prolonged culture, in contrast to the expected effect of immortalization, hFOB acquired a delayed growth rate. In close resemblance to bmMSC at increasing passages, also hFOB showed morphological abnormalities, enlargement and finally reduced proliferation rates together with enhanced expression of the cell cycle inhibitors p21 and p16. hFOB not only have the ability to undergo multilineage differentiation but portray several important aspects of human bone marrow mesenchymal stromal cells. Superior to primary MSC and osteoblasts, hFOB enabled the generation of continuous cell lines. These provide an advanced basis for investigating age-related dysfunctions of MSCs in an in vitro 3D-stem cell microenvironment.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts.Mol Biol Rep. 2011 Nov;38(8):5161-8. doi: 10.1007/s11033-010-0665-2. Epub 2010 Dec 29. Mol Biol Rep. 2011. PMID: 21188535
-
Multilineage differentiation and characterization of the human fetal osteoblastic 1.19 cell line: a possible in vitro model of human mesenchymal progenitors.Stem Cells. 2007 Jan;25(1):125-31. doi: 10.1634/stemcells.2006-0295. Stem Cells. 2007. PMID: 17204605
-
Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway.Eur Rev Med Pharmacol Sci. 2018 Jun;22(12):3962-3970. doi: 10.26355/eurrev_201806_15280. Eur Rev Med Pharmacol Sci. 2018. PMID: 29949171
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
-
Differentiation of osteoblasts and osteocytes from mesenchymal stem cells.Curr Stem Cell Res Ther. 2008 May;3(2):131-45. doi: 10.2174/157488808784223032. Curr Stem Cell Res Ther. 2008. PMID: 18473879 Review.
Cited by
-
Comparison of Primary Human Osteoblast-like Cells and hFOB 1.19 Cells: Contrasting Effects of Proinflammatory Cytokines.Cells. 2025 Aug 15;14(16):1264. doi: 10.3390/cells14161264. Cells. 2025. PMID: 40862744 Free PMC article.
-
A Poly-(ethylene glycol)-diacrylate 3D-Printed Micro-Bioreactor for Direct Cell Biological Implant-Testing on the Developing Chicken Chorioallantois Membrane.Micromachines (Basel). 2022 Jul 31;13(8):1230. doi: 10.3390/mi13081230. Micromachines (Basel). 2022. PMID: 36014153 Free PMC article.
-
Osteogenic Activity and Bone Matrix Mineralization Induced by Vitis vinifera Leaves Extract in Human Osteoblastic Cells.Food Sci Nutr. 2025 Aug 17;13(8):e70785. doi: 10.1002/fsn3.70785. eCollection 2025 Aug. Food Sci Nutr. 2025. PMID: 40831944 Free PMC article.
-
Characterization of hFOB 1.19 Cell Line for Studying Zn-Based Degradable Metallic Biomaterials.Materials (Basel). 2024 Feb 16;17(4):915. doi: 10.3390/ma17040915. Materials (Basel). 2024. PMID: 38399166 Free PMC article.
-
Low-Cost Devices for Three-Dimensional Cell Aggregation, Real-Time Monitoring Microscopy, Microfluidic Immunostaining, and Deconvolution Analysis.Bioengineering (Basel). 2022 Feb 3;9(2):60. doi: 10.3390/bioengineering9020060. Bioengineering (Basel). 2022. PMID: 35200413 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

