Autopsy-diagnosed neurodegenerative dementia cases support the use of cerebrospinal fluid protein biomarkers in the diagnostic work-up
- PMID: 34035398
- PMCID: PMC8149718
- DOI: 10.1038/s41598-021-90366-5
Autopsy-diagnosed neurodegenerative dementia cases support the use of cerebrospinal fluid protein biomarkers in the diagnostic work-up
Abstract
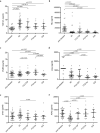
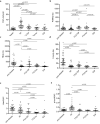
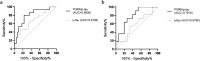
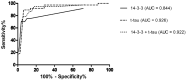
Various proteins play a decisive role in the pathology of different neurodegenerative diseases. Nonetheless, most of these proteins can only be detected during a neuropathological assessment, although some non-specific biomarkers are routinely tested for in the cerebrospinal fluid (CSF) as a part of the differential diagnosis of dementia. In antemortem CSF samples from 117 patients with different types of neuropathologically confirmed neurodegenerative disease with dementia, we assessed total-tau (t-tau), phosphorylated-tau (181P) (p-tau), amyloid-beta (1-42) (Aβ42), TAR DNA binding protein (TDP)-43, progranulin (PGRN), and neurofilament light (NfL) chain levels, and positivity of protein 14-3-3. We found t-tau levels and the t-tau/p-tau ratios were significantly higher in prion diseases compared to the other neurodegenerative diseases. Statistically significant differences in the t-tau/Aβ42 ratio predominantly corresponded to t-tau levels in prion diseases and Aβ42 levels in AD. TDP-43 levels were significantly lower in prion diseases. Additionally, the TDP-43/Aβ42 ratio was better able to distinguish Alzheimer's disease from other neurodegenerative diseases compared to using Aβ42 alone. In frontotemporal lobar degeneration, PRGN levels were significantly higher in comparison to other neurodegenerative diseases. There is an increasing need for biomarkers suitable for diagnostic workups for neurodegenerative diseases. It appears that adding TDP-43 and PGRN to the testing panel for neurodegenerative diseases could improve the resolution of differential diagnoses.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

