Chemical tools for dissecting cell division
- PMID: 34035515
- PMCID: PMC10157795
- DOI: 10.1038/s41589-021-00798-3
Chemical tools for dissecting cell division
Abstract
Components of the cell division machinery typically function at varying cell cycle stages and intracellular locations. To dissect cellular mechanisms during the rapid division process, small-molecule probes act as complementary approaches to genetic manipulations, with advantages of temporal and in some cases spatial control and applicability to multiple model systems. This Review focuses on recent advances in chemical probes and applications to address select questions in cell division. We discuss uses of both enzyme inhibitors and chemical inducers of dimerization, as well as emerging techniques to promote future investigations. Overall, these concepts may open new research directions for applying chemical probes to advance cell biology.
Figures
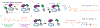

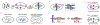
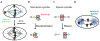
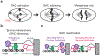
Similar articles
-
Dissecting the mechanisms of cell division.J Biol Chem. 2019 Jul 26;294(30):11382-11390. doi: 10.1074/jbc.AW119.008149. Epub 2019 Jun 7. J Biol Chem. 2019. PMID: 31175154 Free PMC article. Review.
-
Chemical genetics: tailoring tools for cell biology.Trends Cell Biol. 2003 May;13(5):270-7. doi: 10.1016/s0962-8924(03)00077-1. Trends Cell Biol. 2003. PMID: 12742171 Review.
-
Lipid Cell Biology: A Focus on Lipids in Cell Division.Annu Rev Biochem. 2018 Jun 20;87:839-869. doi: 10.1146/annurev-biochem-062917-012448. Epub 2018 Mar 1. Annu Rev Biochem. 2018. PMID: 29494237 Review.
-
Unraveling cell division mechanisms with small-molecule inhibitors.Nat Chem Biol. 2006 Jan;2(1):19-27. doi: 10.1038/nchembio757. Nat Chem Biol. 2006. PMID: 16408087 Review.
-
Current concepts in neuro-oncology: the cell cycle--a review.Neurosurgery. 1997 May;40(5):1000-13; discussion 1013-5. doi: 10.1097/00006123-199705000-00025. Neurosurgery. 1997. PMID: 9149259 Review.
Cited by
-
Abraham Patchornik: The Contemporary Relevance of His Work for Chemistry and Biology.JACS Au. 2024 Dec 20;5(1):3-16. doi: 10.1021/jacsau.4c00779. eCollection 2025 Jan 27. JACS Au. 2024. PMID: 39886589 Free PMC article. Review.
-
Proteome-Wide Profiling of the Covalent-Druggable Cysteines with a Structure-Based Deep Graph Learning Network.Research (Wash D C). 2022 Jul 21;2022:9873564. doi: 10.34133/2022/9873564. eCollection 2022. Research (Wash D C). 2022. PMID: 35958111 Free PMC article.
-
Deterministic Single Cell Encapsulation in Asymmetric Microenvironments to Direct Cell Polarity.Adv Sci (Weinh). 2023 Jan;10(3):e2206014. doi: 10.1002/advs.202206014. Epub 2022 Dec 1. Adv Sci (Weinh). 2023. PMID: 36453581 Free PMC article.
-
Oral administration of tea-derived exosome-like nanoparticles protects epithelial and immune barrier of intestine from psychological stress.Heliyon. 2024 Aug 23;10(17):e36812. doi: 10.1016/j.heliyon.2024.e36812. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39281430 Free PMC article.
References
-
- Sansregret L, Vanhaesebroeck B & Swanton C Determinants and clinical implications of chromosomal instability in cancer. Nat. Rev. Clin. Oncol 15, 139–50 (2018). - PubMed
-
- Leibowitz ML, Zhang C-Z & Pellman D Chromothripsis: A New Mechanism for Rapid Karyotype Evolution. Annu. Rev. Genet 49, 183–211 (2015). - PubMed
-
- Knouse KA, Davoli T, Elledge SJ & Amon A Aneuploidy in Cancer: Seq-ing Answers to Old Questions. Annu. Rev. Cancer Biol 1, 335–54 (2017).
-
- Choi J, Chen J, Schreiber SL & Clardy J Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 273, 239–242 (1996). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

