Development of a rapid and sensitive quantum dot nanobead-based double-antigen sandwich lateral flow immunoassay and its clinical performance for the detection of SARS-CoV-2 total antibodies
- PMID: 34035562
- PMCID: PMC8137357
- DOI: 10.1016/j.snb.2021.130139
Development of a rapid and sensitive quantum dot nanobead-based double-antigen sandwich lateral flow immunoassay and its clinical performance for the detection of SARS-CoV-2 total antibodies
Abstract
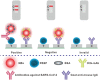
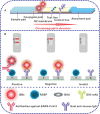
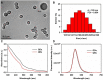
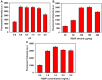
Owing to the over-increasing demands in resisting and managing the coronavirus disease 2019 (COVID-19) pandemic, development of rapid, highly sensitive, accurate, and versatile tools for monitoring total antibody concentrations at the population level has been evolved as an urgent challenge on measuring the fatality rate, tracking the changes in incidence and prevalence, comprehending medical sequelae after recovery, as well as characterizing seroprevalence and vaccine coverage. To this end, herein we prepared highly luminescent quantum dot nanobeads (QBs) by embedding numerous quantum dots into polymer matrix, and then applied it as a signal-amplification label in lateral flow immunoassay (LFIA). After covalently linkage with the expressed recombinant SARS-CoV-2 spike protein (RSSP), the synthesized QBs were used to determine the total antibody levels in sera by virtue of a double-antigen sandwich immunoassay. Under the developed condition, the QB-LFIA can allow the rapid detection of SARS-CoV-2 total antibodies within 15 min with about one order of magnitude improvement in analytical sensitivity compared to conventional gold nanoparticle-based LFIA. In addition, the developed QB-LFIA performed well in clinical study in dynamic monitoring of serum antibody levels in the whole course of SARS-CoV-2 infection. In conclusion, we successfully developed a promising fluorescent immunological sensing tool for characterizing the host immune response to SARS-CoV-2 infection and confirming the acquired immunity to COVID-19 by evaluating the SRAS-CoV-2 total antibody level in the crowd.
Keywords: Fluorescent detection; Lateral flow immunoassay; Quantum dot nanobeads; SARS-CoV-2; Total antibodies.
© 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures
Similar articles
-
Magnetic/fluorescent dual-modal lateral flow immunoassay based on multifunctional nanobeads for rapid and accurate SARS-CoV-2 nucleocapsid protein detection.Anal Chim Acta. 2022 Nov 15;1233:340486. doi: 10.1016/j.aca.2022.340486. Epub 2022 Oct 7. Anal Chim Acta. 2022. PMID: 36283777 Free PMC article.
-
Development of receptor binding domain-based double-antigen sandwich lateral flow immunoassay for the detection and evaluation of SARS-CoV-2 neutralizing antibody in clinical sera samples compared with the conventional virus neutralization test.Talanta. 2023 Apr 1;255:124200. doi: 10.1016/j.talanta.2022.124200. Epub 2022 Dec 21. Talanta. 2023. PMID: 36565525 Free PMC article.
-
Quantum dots assembly enhanced and dual-antigen sandwich structured lateral flow immunoassay of SARS-CoV-2 antibody with simultaneously high sensitivity and specificity.Biosens Bioelectron. 2022 Feb 15;198:113810. doi: 10.1016/j.bios.2021.113810. Epub 2021 Nov 17. Biosens Bioelectron. 2022. PMID: 34840014 Free PMC article.
-
Development and Efficacy of Lateral Flow Point-of-Care Testing Devices for Rapid and Mass COVID-19 Diagnosis by the Detections of SARS-CoV-2 Antigen and Anti-SARS-CoV-2 Antibodies.Diagnostics (Basel). 2021 Sep 24;11(10):1760. doi: 10.3390/diagnostics11101760. Diagnostics (Basel). 2021. PMID: 34679458 Free PMC article. Review.
-
Recent Advances in Quantum Dot-Based Lateral Flow Immunoassays for the Rapid, Point-of-Care Diagnosis of COVID-19.Biosensors (Basel). 2023 Aug 3;13(8):786. doi: 10.3390/bios13080786. Biosensors (Basel). 2023. PMID: 37622872 Free PMC article. Review.
Cited by
-
Sensitization Strategies of Lateral Flow Immunochromatography for Gold Modified Nanomaterials in Biosensor Development.Int J Nanomedicine. 2023 Dec 21;18:7847-7863. doi: 10.2147/IJN.S436379. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38146466 Free PMC article. Review.
-
A Universal Fluorescent Immunochromatography Assay Based on Quantum Dot Nanoparticles for the Rapid Detection of Specific Antibodies against SARS-CoV-2 Nucleocapsid Protein.Int J Mol Sci. 2022 Jun 2;23(11):6225. doi: 10.3390/ijms23116225. Int J Mol Sci. 2022. PMID: 35682904 Free PMC article.
-
Merging microfluidics with luminescence immunoassays for urgent point-of-care diagnostics of COVID-19.Trends Analyt Chem. 2022 Dec;157:116814. doi: 10.1016/j.trac.2022.116814. Epub 2022 Nov 7. Trends Analyt Chem. 2022. PMID: 36373139 Free PMC article. Review.
-
Magnetic/fluorescent dual-modal lateral flow immunoassay based on multifunctional nanobeads for rapid and accurate SARS-CoV-2 nucleocapsid protein detection.Anal Chim Acta. 2022 Nov 15;1233:340486. doi: 10.1016/j.aca.2022.340486. Epub 2022 Oct 7. Anal Chim Acta. 2022. PMID: 36283777 Free PMC article.
-
Application of microfluidic technologies on COVID-19 diagnosis and drug discovery.Acta Pharm Sin B. 2023 Feb 24;13(7):2877-96. doi: 10.1016/j.apsb.2023.02.014. Online ahead of print. Acta Pharm Sin B. 2023. PMID: 36855672 Free PMC article. Review.
References
-
- WHO. Coronavirus disease (COVID-19) pandemic. Revised January 16, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

