Assessment of the Vanillin Anti-Inflammatory and Regenerative Potentials in Inflamed Primary Human Gingival Fibroblast
- PMID: 34035660
- PMCID: PMC8116147
- DOI: 10.1155/2021/5562340
Assessment of the Vanillin Anti-Inflammatory and Regenerative Potentials in Inflamed Primary Human Gingival Fibroblast
Abstract
Background: Inflammatory responses have been associated with delayed oral mucosal wound healing and the pathogenesis of the periodontal disease. The invasion of microbes into the tissues and the establishment of a chronic infection may be due to impaired healing. The protracted inflammatory phase may delay wound healing and probably support tissue fibrosis and reduce tissue regeneration. Vanillin is a well-known natural compound with potential anti-inflammatory capacity. Hence, we hypothesized that Vanillin could accelerate wound healing reducing inflammation and especially cytokine production making the oral tissue repair process easier.
Methods: Our hypothesis was tested using primary human gingival fibroblast (HGF) cell pretreated with Vanillin and primed with IL-1β, as inductor of proinflammatory environment. After 24 hours of treatments, the gene expression and production of IL-6, TNF-α, IL-8, COX-2, iNOS, and nitric oxide (NO) generation and the wound healing rate were determined.
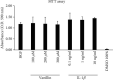
Results: In IL-1β-primed cells, preincubation with Vanillin reduced IL-6, IL-8, COX-2, and iNOS expression and NO release, compared to IL-1β-primed cells. Moreover, Vanillin determines the increased gene expression of nAChRα7, leading us to hypothesize a role of Vanillin in the activation of the cholinergic anti-inflammatory pathway. Furthermore, in presence of mechanical injury, the Vanillin preincubation, wound closure may be reducing the expression and release of IL-6 and TNF-α and upregulation of COX-2 and IL-8.
Conclusion: Together, the results of this study highlight the anti-inflammatory and tissue repair ability of Vanillin in IL-1β-primed HGF. Therefore, Vanillin shows a potential therapeutic interest as an inflammatory modulator molecule with novel application in periodontal regeneration and oral health.
Copyright © 2021 Erica Costantini et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
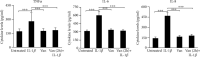








References
-
- Palombo E. A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evidence-based complementary and Alternative Medicine. 2011;2011:15. doi: 10.1093/ecam/nep067.680354 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

