Comparison of two immunoassays for the measurement of serum HE4 for ovarian cancer
- PMID: 34036134
- PMCID: PMC8138765
- DOI: 10.1016/j.plabm.2021.e00235
Comparison of two immunoassays for the measurement of serum HE4 for ovarian cancer
Abstract
Introduction: The use of Human Epididymis Protein 4 (HE4) as a biomarker for ovarian cancer is gaining traction, providing the impetus for development of a high throughput automated HE4 assay that is comparable to the conventional manual enzyme immunometric-assay (EIA). The aim of this study was to compare two immunoassay methods for the measurement of serum HE4.
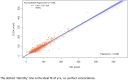
Materials and methods: 1348 serum samples were analysed for serum HE4 using both the EIA and the automated chemiluminescent immunoassay (CLEIA) methods. HE4 values were compared using a Passing-Bablok regression and agreement assessed using Lin's concordance correlation coefficient (CCC). The absolute and percentage bias of the CLEIA compared to EIA was determined.
Results: There was moderate agreement between the two methods (CCC 0.929, 95%CI 0.923-0.936). Passing-Bablok regression demonstrated an overestimation of the CLEIA [constant 4.44 (95%CI 2.96-5.68), slope 1.04 (95%CI 1.02-1.07)]. The CLEIA method had a mean percentage bias of 16.25% compared to the EIA method.
Conclusion: The CLEIA significantly overestimated serum HE4 values compared to the EIA, which could impact clinical interpretation and patient management. Further studies are required to develop an appropriate cut-off depending on the population being investigated and the analytic method being used.
Keywords: Biomarker; HE4; Immunoassay; Ovarian cancer detection.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interests.
Figures
Similar articles
-
Verification of the harmonization of human epididymis protein 4 assays.Clin Chem Lab Med. 2016 Oct 1;54(10):1635-43. doi: 10.1515/cclm-2015-1142. Clin Chem Lab Med. 2016. PMID: 27028735
-
HE4 and epithelial ovarian cancer: comparison and clinical evaluation of two immunoassays and a combination algorithm.Clin Chim Acta. 2011 Jul 15;412(15-16):1447-53. doi: 10.1016/j.cca.2011.04.028. Epub 2011 Apr 30. Clin Chim Acta. 2011. PMID: 21557935
-
The reference intervals for HE4, CA125 and ROMA in healthy female with electrochemiluminescence immunoassay.Clin Biochem. 2013 Nov;46(16-17):1705-8. doi: 10.1016/j.clinbiochem.2013.08.019. Epub 2013 Sep 6. Clin Biochem. 2013. PMID: 24012857
-
The diagnostic accuracy of human epididymis protein 4 (HE4) for discriminating between benign and malignant pelvic masses: a systematic review and meta-analysis.Acta Obstet Gynecol Scand. 2021 Oct;100(10):1788-1799. doi: 10.1111/aogs.14224. Epub 2021 Jul 19. Acta Obstet Gynecol Scand. 2021. PMID: 34212386
-
Standard and optimal cut-off values of serum ca-125, HE4 and ROMA in preoperative prediction of ovarian cancer in Vietnam.Gynecol Oncol Rep. 2018 Jul 18;25:110-114. doi: 10.1016/j.gore.2018.07.002. eCollection 2018 Aug. Gynecol Oncol Rep. 2018. PMID: 30109256 Free PMC article. Review.
Cited by
-
The Performance of HE4 Alone and in Combination with CA125 for the Detection of Ovarian Cancer in an Enriched Primary Care Population.Cancers (Basel). 2022 Apr 24;14(9):2124. doi: 10.3390/cancers14092124. Cancers (Basel). 2022. PMID: 35565253 Free PMC article.
-
Protein Glycosylation as Biomarkers in Gynecologic Cancers.Diagnostics (Basel). 2022 Dec 15;12(12):3177. doi: 10.3390/diagnostics12123177. Diagnostics (Basel). 2022. PMID: 36553184 Free PMC article. Review.
-
HE4 as a Biomarker for Endometrial Cancer.Cancers (Basel). 2021 Sep 23;13(19):4764. doi: 10.3390/cancers13194764. Cancers (Basel). 2021. PMID: 34638250 Free PMC article. Review.
-
Evaluation of the Comparability of Wantai Wan200+ Instrument with Routine Laboratory Assays for 21 Different Analytes.J Clin Med. 2024 Apr 12;13(8):2246. doi: 10.3390/jcm13082246. J Clin Med. 2024. PMID: 38673517 Free PMC article.
-
Urine CA125 and HE4 for the Detection of Ovarian Cancer in Symptomatic Women.Cancers (Basel). 2023 Feb 16;15(4):1256. doi: 10.3390/cancers15041256. Cancers (Basel). 2023. PMID: 36831601 Free PMC article.
References
-
- Ferraro S., Borille S., Carnevale A., Frusciante E., Bassani N., Panteghini M. Verification of the harmonization of human epididymis protein 4 assays. Clin. Chem. Lab. Med. 2016;54(10):1635–1643. - PubMed
-
- Huang J., Chen J., Huang Q. Diagnostic value of HE4 in ovarian cancer: a meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018;231:35–42. - PubMed
-
- Dayyani F., Uhlig S., Colson B., Simon K., Rolny V., Morgenstern D. Diagnostic performance of risk of ovarian malignancy Algorithm against CA125 and HE4 in connection with ovarian cancer: a meta-analysis. Int. J. Gynecol. Canc. 2016;26(9):1586–1593. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials