Integrating Clinical and Polygenic Factors to Predict Breast Cancer Risk in Women Undergoing Genetic Testing
- PMID: 34036224
- PMCID: PMC8140787
- DOI: 10.1200/PO.20.00246
Integrating Clinical and Polygenic Factors to Predict Breast Cancer Risk in Women Undergoing Genetic Testing
Abstract
Purpose: Screening and prevention decisions for women at increased risk of developing breast cancer depend on genetic and clinical factors to estimate risk and select appropriate interventions. Integration of polygenic risk into clinical breast cancer risk estimators can improve discrimination. However, correlated genetic effects must be incorporated carefully to avoid overestimation of risk.
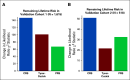
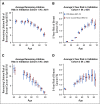
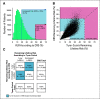
Materials and methods: A novel Fixed-Stratified method was developed that accounts for confounding when adding a new factor to an established risk model. A combined risk score (CRS) of an 86-single-nucleotide polymorphism polygenic risk score and the Tyrer-Cuzick v7.02 clinical risk estimator was generated with attenuation for confounding by family history. Calibration and discriminatory accuracy of the CRS were evaluated in two independent validation cohorts of women of European ancestry (N = 1,615 and N = 518). Discrimination for remaining lifetime risk was examined by age-adjusted logistic regression. Risk stratification with a 20% risk threshold was compared between CRS and Tyrer-Cuzick in an independent clinical cohort (N = 32,576).
Results: Simulation studies confirmed that the Fixed-Stratified method produced accurate risk estimation across patients with different family history. In both validation studies, CRS and Tyrer-Cuzick were significantly associated with breast cancer. In an analysis with both CRS and Tyrer-Cuzick as predictors of breast cancer, CRS added significant discrimination independent of that captured by Tyrer-Cuzick (P < 10-11 in validation 1; P < 10-7 in validation 2). In an independent cohort, 18% of women shifted breast cancer risk categories from their Tyrer-Cuzick-based risk compared with risk estimates by CRS.
Conclusion: Integrating clinical and polygenic factors into a risk model offers more effective risk stratification and supports a personalized genomic approach to breast cancer screening and prevention.
© 2021 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Elisha HughesEmployment: Myriad Genetics Stock and Other Ownership Interests: Myriad GeneticsPlacede TshiabaEmployment: Myriad Genetics Stock and Other Ownership Interests: Myriad GeneticsSusanne WagnerEmployment: Myriad Genetics Stock and Other Ownership Interests: Myriad Genetics Patents, Royalties, Other Intellectual Property: Coauthor of patents held by Myriad Genetics, no royaltiesThaddeus JudkinsEmployment: Myriad Genetics Stock and Other Ownership Interests: Myriad Genetics Travel, Accommodations, Expenses: Myriad GeneticsEric RosenthalEmployment: Myriad Genetics Stock and Other Ownership Interests: Myriad GeneticsBenjamin RoaEmployment: Myriad Genetics Leadership: Myriad Genetics Stock and Other Ownership Interests: Myriad Genetics Research Funding: Myriad Genetics Patents, Royalties, Other Intellectual Property: Intellectual property held by employer Myriad Genetics Travel, Accommodations, Expenses: Myriad GeneticsShannon GallagherEmployment: Myriad Genetics Stock and Other Ownership Interests: Myriad GeneticsStephanie MeekEmployment: Myriad Genetics Stock and Other Ownership Interests: Myriad GeneticsKathryn DaltonSpeakers' Bureau: Myriad GeneticsDanna F. GrearConsulting or Advisory Role: Myriad Genetics Speakers' Bureau: Myriad Genetics Travel, Accommodations, Expenses: Myriad GeneticsSusan M. DomchekHonoraria: AstraZeneca, Clovis Oncology, Bristol-Myers Squibb Research Funding: AstraZeneca, Clovis OncologyJudy GarberConsulting or Advisory Role: Novartis, GTx, Helix BioPharma, Konica Minolta, Aleta BioTherapeutics, H3 Biomedicine, Kronos Bio Research Funding: Novartis, Ambry Genetics, InVitae, Myriad Genetics Other Relationship: Susan G. Komen for the Cure, AACR, Diana Helis Henry Medical Foundation, James P. Wilmot Foundation, Adrienne Helis Malvin Medical Research Foundation, Breast Cancer Research Foundation, Facing our Risk of Cancer EmpoweredJohnathan M. LancasterEmployment: Myriad Genetics, Regeneron Leadership: Myriad Genetics, Regeneron Stock and Other Ownership Interests: Myriad Genetics, RegeneronJeffrey N. WeitzelSpeakers' Bureau: AstraZenecaAllison W. KurianResearch Funding: Myriad Genetics Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, GenentechJerry S. LanchburyEmployment: Myriad Genetics Leadership: Myriad Genetics Stock and Other Ownership Interests: Myriad Genetics Patents, Royalties, Other Intellectual Property: I am an inventor on multiple patents filed by Myriad Genetics Travel, Accommodations, Expenses: Myriad GeneticsAlexander GutinEmployment: Myriad Genetics Stock and Other Ownership Interests: Myriad Genetics, Gilead SciencesMark E. RobsonConsulting or Advisory Role: Change HealthCare Research Funding: AstraZeneca, AbbVie, Pfizer, Merck Travel, Accommodations, Expenses: AstraZeneca, Merck Other Relationship: Research to Practice, Clinical Care Options, Physicians' Education Resource, Invitae, Pfizer (OPTIONAL) Uncompensated Relationships: Merck, Pfizer, Daiichi Sankyo, Epic Sciences, https://openpaymentsdata.cms.gov/physician/612669/summary No other potential conflicts of interest were reported.
Figures

Similar articles
-
Comprehensive Breast Cancer Risk Assessment for CHEK2 and ATM Pathogenic Variant Carriers Incorporating a Polygenic Risk Score and the Tyrer-Cuzick Model.JCO Precis Oncol. 2021 Jun 24;5:PO.20.00484. doi: 10.1200/PO.20.00484. eCollection 2021 Jun. JCO Precis Oncol. 2021. PMID: 34322652 Free PMC article.
-
Comparative validation of the BOADICEA and Tyrer-Cuzick breast cancer risk models incorporating classical risk factors and polygenic risk in a population-based prospective cohort of women of European ancestry.Breast Cancer Res. 2021 Feb 15;23(1):22. doi: 10.1186/s13058-021-01399-7. Breast Cancer Res. 2021. PMID: 33588869 Free PMC article.
-
Validation of a clinical breast cancer risk assessment tool combining a polygenic score for all ancestries with traditional risk factors.Genet Med. 2024 Jul;26(7):101128. doi: 10.1016/j.gim.2024.101128. Epub 2024 Jun 3. Genet Med. 2024. PMID: 38829299
-
Risk and risk assessment for breast cancer: molecular and clinical aspects.Maturitas. 2007 May 20;57(1):56-60. doi: 10.1016/j.maturitas.2007.02.013. Epub 2007 Mar 26. Maturitas. 2007. PMID: 17386982 Review.
-
Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field.Breast Cancer Res. 2020 Feb 17;22(1):21. doi: 10.1186/s13058-020-01260-3. Breast Cancer Res. 2020. PMID: 32066492 Free PMC article. Review.
Cited by
-
Primary care physician use of patient race and polygenic risk scores in medical decision-making.Genet Med. 2023 Apr;25(4):100800. doi: 10.1016/j.gim.2023.100800. Epub 2023 Feb 4. Genet Med. 2023. PMID: 36748708 Free PMC article. Clinical Trial.
-
Deconstructing a Syndrome: Genomic Insights Into PCOS Causal Mechanisms and Classification.Endocr Rev. 2022 Nov 25;43(6):927-965. doi: 10.1210/endrev/bnac001. Endocr Rev. 2022. PMID: 35026001 Free PMC article.
-
Information needs on breast cancer genetic and non-genetic risk factors in relatives of women with a BRCA1/2 or PALB2 pathogenic variant.Breast. 2021 Dec;60:38-44. doi: 10.1016/j.breast.2021.08.011. Epub 2021 Aug 23. Breast. 2021. PMID: 34455229 Free PMC article.
-
Combining rare and common genetic variants improves population risk stratification for breast cancer.Genet Med Open. 2024 Feb 2;2:101826. doi: 10.1016/j.gimo.2024.101826. eCollection 2024. Genet Med Open. 2024. PMID: 39669587 Free PMC article.
-
Translational Genomic Research: The Association between Genetic Profiles and Cognitive Functioning or Cardiac Function Among Breast Cancer Survivors Completing Chemotherapy.Biol Res Nurs. 2022 Oct;24(4):433-447. doi: 10.1177/10998004221094386. Epub 2022 May 2. Biol Res Nurs. 2022. PMID: 35499926 Free PMC article.
References
-
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation Ann Intern Med 164244–2552016 - PubMed
-
- Daly MB, Pilarski R, Berry M, et al. NCCN Clinical Practice Guidelines in Oncology, Genetic/Familial High-Risk Assessment: Breast and ovarian (version 3.2019) NCCN Clinical Practice Guidelines in Oncology. 2019 https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

