MET Exon 14 Skipping Mutations in Non-Small-Cell Lung Cancer: An Overview of Biology, Clinical Outcomes, and Testing Considerations
- PMID: 34036238
- PMCID: PMC8140815
- DOI: 10.1200/PO.20.00516
MET Exon 14 Skipping Mutations in Non-Small-Cell Lung Cancer: An Overview of Biology, Clinical Outcomes, and Testing Considerations
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center. Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments). Mark A. SocinskiSpeakers' Bureau: AstraZeneca, Bristol-Myers Squibb, Celgene, Genentech, Merck, Novartis Research Funding: AstraZeneca, Bristol-Myers Squibb, Genentech, Merck, Novartis, TakedaNathan A. PennellConsulting or Advisory Role: Amgen, AstraZeneca, Bristol-Myers Squibb, Cota, Eli Lilly, Genentech, Inivata, Merck, Pfizer Open Payments Link: https://openpaymentsdata.cms.gov/physician/204570/summaryKurtis D. DaviesConsulting or Advisory Role: Novartis Travel, Accommodations, Expenses: Archer No other potential conflicts of interest were reported.
Figures
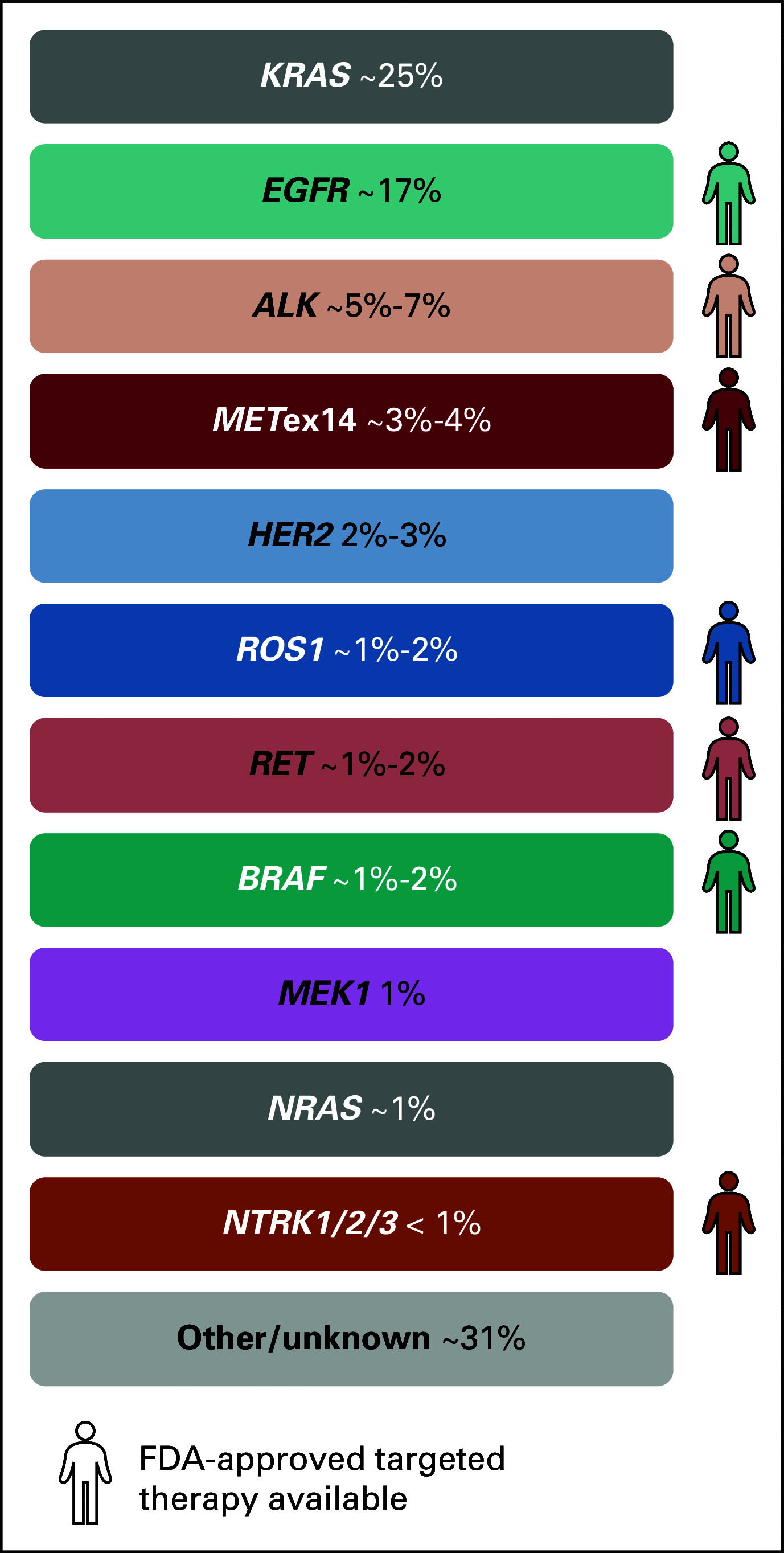

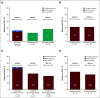

References
-
- Siegel RL, Miller KD, Jemal A.Cancer statistics, 2020 CA Cancer J Clin 707–302020 - PubMed
-
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: Current therapies and new targeted treatments Lancet 389299–3112017 - PubMed
-
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors Cancer Discov 5850–8592015 - PubMed
-
- American Cancer Society About lung cancer. https://www.cancer.org/cancer/lung-cancer/about.html
-
- National Cancer Institute Drugs approved for lung cancer. https://www.cancer.gov/about-cancer/treatment/drugs/lung
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

