A human liver chimeric mouse model for non-alcoholic fatty liver disease
- PMID: 34036256
- PMCID: PMC8138774
- DOI: 10.1016/j.jhepr.2021.100281
A human liver chimeric mouse model for non-alcoholic fatty liver disease
Abstract
Background & aims: The accumulation of neutral lipids within hepatocytes underlies non-alcoholic fatty liver disease (NAFLD), which affects a quarter of the world's population and is associated with hepatitis, cirrhosis, and hepatocellular carcinoma. Despite insights gained from both human and animal studies, our understanding of NAFLD pathogenesis remains limited. To better study the molecular changes driving the condition we aimed to generate a humanised NAFLD mouse model.
Methods: We generated TIRF (transgene-free Il2rg -/-/Rag2 -/-/Fah -/-) mice, populated their livers with human hepatocytes, and fed them a Western-type diet for 12 weeks.
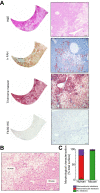
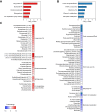
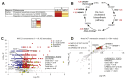
Results: Within the same chimeric liver, human hepatocytes developed pronounced steatosis whereas murine hepatocytes remained normal. Unbiased metabolomics and lipidomics revealed signatures of clinical NAFLD. Transcriptomic analyses showed that molecular responses diverged sharply between murine and human hepatocytes, demonstrating stark species differences in liver function. Regulatory network analysis indicated close agreement between our model and clinical NAFLD with respect to transcriptional control of cholesterol biosynthesis.
Conclusions: These NAFLD xenograft mice reveal an unexpected degree of evolutionary divergence in food metabolism and offer a physiologically relevant, experimentally tractable model for studying the pathogenic changes invoked by steatosis.
Lay summary: Fatty liver disease is an emerging health problem, and as there are no good experimental animal models, our understanding of the condition is poor. We here describe a novel humanised mouse system and compare it with clinical data. The results reveal that the human cells in the mouse liver develop fatty liver disease upon a Western-style fatty diet, whereas the mouse cells appear normal. The molecular signature (expression profiles) of the human cells are distinct from the mouse cells and metabolic analysis of the humanised livers mimic the ones observed in humans with fatty liver. This novel humanised mouse system can be used to study human fatty liver disease.
Keywords: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBPEGs, cholesterol biosynthesis pathway enzyme genes; CE, cholesteryl ester; CER, ceramide; CHHs, chimeric human hepatocytes; CMHs, chimeric mouse hepatocytes; CT, confidence transcript; DAG, diacylglycerol; DCER, dihydroceramide; DEG, differentially expressed gene; FA, fatty acid; FAH, fumarylacetoacetate hydrolase; FFA, free fatty acid; GGT, gamma-glutamyl transpeptidase; HCC, hepatocellular carcinoma; HCER, hexosylceramide; HCT, high confidence transcriptional target; Human disease modelling; Humanised mice; LCER, lactosylceramide; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; Lipid metabolism; MAG, monoacylglycerol; MUFA, monounsaturated fatty acid; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NC, normal chow; NTBC, nitisinone; Non-alcoholic fatty liver disease; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PNPLA3, patatin-like-phospholipase domain-containing protein 3; PUFA, polyunsaturated free FA; SM, sphingomyelin; SREBP, sterol regulatory element-binding protein; Steatosis; TAG, triacylglycerol; TIRF, transgene-free Il2rg-/-/Rag2-/-/Fah-/-; WD, Western-type diet; hALB, human albumin.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no personal or financial conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease – meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. - PubMed
-
- Font-Burgada J., Sun B., Karin M. Obesity and cancer: the oil that feeds the flame. Cell Metab. 2016;23:48–62. - PubMed
-
- Hebbard L., George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35–44. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

