Effects of Txk‑mediated activation of NF‑κB signaling pathway on neurological deficit and oxidative stress after ischemia‑reperfusion in rats
- PMID: 34036382
- PMCID: PMC8160475
- DOI: 10.3892/mmr.2021.12163
Effects of Txk‑mediated activation of NF‑κB signaling pathway on neurological deficit and oxidative stress after ischemia‑reperfusion in rats
Abstract
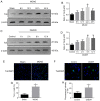
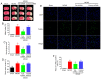
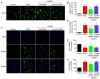
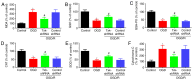
Ischemic stroke is an extremely mortal cerebrovascular disease, and neuroinflammation and oxidative stress emerge as important traits of ischemic stroke. However, as an inflammation‑associated factor, Txk tyrosine kinases (Txk) has been poorly studied in neuroscience research. The aim of the present study was to investigate the role of Txk after ischemia‑reperfusion (I/R) in vivo and in vitro, observe the association between Txk knockdown and neurological deficit and oxidative stress, and to explore whether the process was mediated by the activation of nuclear factor (NF)‑κB signaling pathway. Middle cerebral artery occlusion (MCAO), oxygen and glucose deprivation/reperfusion (OGD/R) model and western blotting have been used to simulate the I/R injury to analyze the expression, and to approximate the localization of Txk, respectively. Brain infarct volume, neurological score, brain water content, apoptosis and oxidative stress assays in vivo and apoptosis, cellular viability, the LDH release and oxidative stress assays in vitro were observed using a Txk‑knockdown lentivirus. Finally, NF‑κB overexpression lentivirus was applied to discuss whether the role of Txk following I/R was regulated by the NF‑κB signaling pathway. The results show that the Txk expression peaked at 24 h after MCAO and 6 h after OGD/R, respectively. Txk molecules gradually entered the nucleus after MCAO and OGD/R. The Txk‑knockdown lentivirus resulted in decreased brain infarct volume, neurological score, brain water content, apoptosis and oxidative stress after MCAO in vivo. Besides, Txk knockdown decreased apoptosis, LDH release, oxidative stress, and increased cellular viability, after ODG in vitro. Finally, NF‑κB overexpression reversed the process of neurological deficit and oxidative stress after Txk regulation in vivo and vitro. Overall, the present study suggests that Txk potentially regulates apoptosis, neurological deficit, and oxidative stress after I/R, by entering the nucleus. NF‑κB maybe the downstream target factor of Txk.
Keywords: Txk; ischemia‑reperfusion; neurological deficit; nuclear factor‑κB; oxidative stress.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Kim JH, Nagy Á, Putzu A, Belletti A, Biondi-Zoccai G, Likhvantsev VV, Yavorovskiy AG, Landoni G. Therapeutic hypothermia in Critically Ill patients: A systematic review and meta-analysis of high quality randomized trials. Crit Care Med. 2020;48:1047–1054. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

