Guan Xin Dan Shen formulation protects db/db mice against diabetic cardiomyopathy via activation of Nrf2 signaling
- PMID: 34036388
- PMCID: PMC8170264
- DOI: 10.3892/mmr.2021.12170
Guan Xin Dan Shen formulation protects db/db mice against diabetic cardiomyopathy via activation of Nrf2 signaling
Abstract
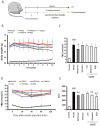
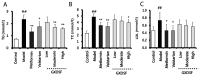
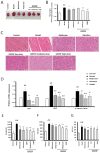
Guan Xin Dan Shen formulation (GXDSF) is a widely used treatment for the management of coronary heart disease in China and is composed of three primary components: Dalbergiae odoriferae Lignum, Salviae miltiorrhizae Radix et Rhizoma and Panax notoginseng Radix et Rhizoma. However, the potential use of GXDSF for the management of diabetic cardiomyopathy (DCM) has not been previously assessed. The present study aimed to assess the effects of GXDSF on DCM, as well as the underlying mechanism. In the present study, db/db mice were used. Following treatment with GXDSF for 10 weeks, fasting blood glucose, insulin sensitivity, serum lipid levels and cardiac enzyme levels were detected. Cardiac pathological alterations and cardiac function were assessed by performing hematoxylin and eosin staining and echocardiograms, respectively. TUNEL assays were conducted to assess cardiomyocyte apoptosis. Additionally, reverse transcription‑quantitative PCR and western blotting were performed to evaluate the expression of apoptosis‑associated genes and proteins, respectively. In the model group, the db/db mice displayed obesity, hyperlipidemia and hyperglycemia, accompanied by noticeable myocardial hypertrophy and diastolic dysfunction. Following treatment with GXDSF for 10 weeks, serum triglyceride levels were lower and insulin sensitivity was enhanced in db/db mice compared with the model group, which indicated improvement in condition. Cardiac hypertrophy and dysfunction were also improved in db/db mice following treatment with GXDSF, resulting in significantly increased left ventricular ejection fraction and fractional shortening compared with the model group. Following treatment with metformin or GXDSF, model‑induced increases in levels of myocardial enzymes were decreased in the moderate and high dose groups. Moreover, the results indicated that, compared with the model group, GXDSF significantly inhibited cardiomyocyte apoptosis in diabetic heart tissues by increasing Bcl‑2 expression and decreasing the expression levels of Bax, cleaved caspase‑3 and cleaved caspase‑9. Mechanistically, GXDSF enhanced Akt phosphorylation, which upregulated antioxidant enzymes mediated by nuclear factor erythroid 2‑related factor 2 (Nrf2) signaling. Collectively, the results of the present study indicated that GXDSF attenuated cardiac dysfunction and inhibited cardiomyocyte apoptosis in diabetic mice via activation of Akt/Nrf2 signaling. Therefore, GXDSF may serve as a potential therapeutic agent for the management of DCM.
Keywords: Guan Xin Dan Shen formulation; diabetes mellitus; diabetic cardiomyopathy; heart; myocardial apoptosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Guanxin Danshen Formulation Protects against Myocardial Ischemia Reperfusion Injury-Induced Left Ventricular Remodeling by Upregulating Estrogen Receptor β.Front Pharmacol. 2017 Nov 1;8:777. doi: 10.3389/fphar.2017.00777. eCollection 2017. Front Pharmacol. 2017. PMID: 29163163 Free PMC article.
-
FNDC5/Irisin attenuates diabetic cardiomyopathy in a type 2 diabetes mouse model by activation of integrin αV/β5-AKT signaling and reduction of oxidative/nitrosative stress.J Mol Cell Cardiol. 2021 Nov;160:27-41. doi: 10.1016/j.yjmcc.2021.06.013. Epub 2021 Jul 3. J Mol Cell Cardiol. 2021. PMID: 34224725
-
[Effect of Guanxin Danshen Formulation on diabetic kidney disease in db/db mice through regulation of Nrf2 pathway].Zhongguo Zhong Yao Za Zhi. 2020 Jun;45(11):2595-2600. doi: 10.19540/j.cnki.cjcmm.20200418.401. Zhongguo Zhong Yao Za Zhi. 2020. PMID: 32627494 Chinese.
-
Novel insights into the central protective role of ACE2 in diabetic cardiomyopathy: from underlying signaling pathways to therapeutic perspectives.Mol Cell Biochem. 2025 Jun;480(6):3535-3551. doi: 10.1007/s11010-024-05196-6. Epub 2025 Feb 10. Mol Cell Biochem. 2025. PMID: 39928210 Review.
-
Role of melatonin in mitigation of insulin resistance and ensuing diabetic cardiomyopathy.Life Sci. 2024 Oct 15;355:122993. doi: 10.1016/j.lfs.2024.122993. Epub 2024 Aug 16. Life Sci. 2024. PMID: 39154810 Review.
Cited by
-
Critical Appraisal of Pharmaceutical Therapy in Diabetic Cardiomyopathy-Challenges and Prospectives.Pharmaceuticals (Basel). 2025 Jan 20;18(1):134. doi: 10.3390/ph18010134. Pharmaceuticals (Basel). 2025. PMID: 39861195 Free PMC article. Review.
-
Reduced Levels of H2S in Diabetes-Associated Osteoarthritis Are Linked to Hyperglycaemia, Nrf-2/HO-1 Signalling Downregulation and Chondrocyte Dysfunction.Antioxidants (Basel). 2022 Mar 25;11(4):628. doi: 10.3390/antiox11040628. Antioxidants (Basel). 2022. PMID: 35453313 Free PMC article.
-
Tongmai Hypoglycemic Capsule Attenuates Myocardial Oxidative Stress and Fibrosis in the Development of Diabetic Cardiomyopathy in Rats.Chin J Integr Med. 2025 Mar;31(3):251-260. doi: 10.1007/s11655-024-4002-3. Epub 2024 Dec 7. Chin J Integr Med. 2025. PMID: 39644459
-
The role of Nrf2 signaling pathway in diabetic cardiomyopathy: from pathogenesis to traditional Chinese medicine interventions.Front Cardiovasc Med. 2025 Jul 14;12:1492499. doi: 10.3389/fcvm.2025.1492499. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40727576 Free PMC article. Review.
References
-
- Barooti A, Kamran M, Kharazmi F, Eftakhar E, Malekzadeh K, Talebi A, Soltani N. Effect of oral magnesium sulfate administration on blood glucose hemostasis via inhibition of gluconeogenesis and FOXO1 gene expression in liver and muscle in diabetic rats. Biomed Pharmacother. 2019;109:1819–1825. doi: 10.1016/j.biopha.2018.10.164. - DOI - PubMed
-
- Tsai TH, Lin CJ, Chua S, Chung SY, Chen SM, Lee CH, Hang CL. Deletion of RasGRF1 attenuated interstitial fibrosis in streptozotocin-induced diabetic cardiomyopathy in mice through affecting inflammation and oxidative stress. Int J Mol Sci. 2018;19:3094. doi: 10.3390/ijms19103094. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

