Mechanical stress influences the morphology and function of human uterosacral ligament fibroblasts and activates the p38 MAPK pathway
- PMID: 34036402
- PMCID: PMC9343297
- DOI: 10.1007/s00192-021-04850-7
Mechanical stress influences the morphology and function of human uterosacral ligament fibroblasts and activates the p38 MAPK pathway
Abstract
Introduction and hypothesis: Pelvic organ prolapse (POP) is a common condition in older women that affects quality of life. Mechanical injury of the pelvic floor support system contributes to POP development. In our study, we aimed to examine the mechanical damage to human uterosacral ligament fibroblasts (hUSLFs) to preliminarily explore the mechanism of mechanical transduction in POP.
Methods: hUSLFs were derived from POP and non-POP patients. Mechanical stress was induced by the FX-5000 T-cell stress loading system. Student's t-test was used for comparisons between different groups.
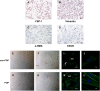
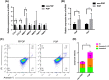
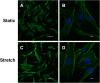
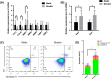
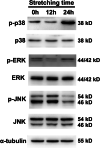
Results: We found that hUSLFs from POP patients were larger and longer than those from non-POP patients and exhibited cytoskeleton F-actin rearrangement. Collagen I and III expression levels were lower and matrix metalloproteinase 1 (MMP1) levels were higher in POP patients than in non-POP patients. Additionally, the apoptosis rate was significantly increased in POP patients compared to non-POP patients. After mechanical stretching, hUSLFs underwent a POP-like transformation. Cells became longer, and the cytoskeleton became thicker and rearranged. The extracellular matrix (ECM) was remodelled because of the upregulation of collagen I and III expression and downregulation of MMP1 expression. Mechanical stress also induced hUSLF apoptosis. Notably, we found that the p38 MAPK pathway was activated by mechanical stretching.
Conclusions: Mechanical stress induced morphological changes in ligament fibroblasts, leading to cytoskeleton and ECM remodelling and cell apoptosis. p38 MAPK might be involved in this process, providing novel insights into the mechanical biology of and possible therapies for this disease.
Keywords: Human uterosacral ligament fibroblasts; Mechanical stress; Pelvic organ prolapse; p38 MAPK pathway.
© 2021. The Author(s).
Conflict of interest statement
None.
Figures
Similar articles
-
Roles and mechanisms of biomechanical-biochemical coupling in pelvic organ prolapse.Front Med (Lausanne). 2024 Feb 12;11:1303044. doi: 10.3389/fmed.2024.1303044. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38410754 Free PMC article. Review.
-
SIRT1 as a potential therapeutic target in pelvic organ prolapse due to protective effects against oxidative stress and cellular senescence in human uterosacral ligament fibroblasts.Neurourol Urodyn. 2024 Jun;43(5):1217-1229. doi: 10.1002/nau.25455. Epub 2024 Apr 1. Neurourol Urodyn. 2024. PMID: 38558173
-
The protective effect of 17 β-estradiol on human uterosacral ligament fibroblasts from postmenopausal women with pelvic organ prolapse.Front Physiol. 2022 Oct 10;13:980843. doi: 10.3389/fphys.2022.980843. eCollection 2022. Front Physiol. 2022. PMID: 36299259 Free PMC article.
-
The role of GPX1 in the pathogenesis of female pelvic organ prolapse.PLoS One. 2017 Aug 7;12(8):e0181896. doi: 10.1371/journal.pone.0181896. eCollection 2017. PLoS One. 2017. PMID: 28783735 Free PMC article.
-
Role of Fibroblasts and Myofibroblasts on the Pathogenesis and Treatment of Pelvic Organ Prolapse.Biomolecules. 2022 Jan 6;12(1):94. doi: 10.3390/biom12010094. Biomolecules. 2022. PMID: 35053242 Free PMC article. Review.
Cited by
-
Advanced glycation end products (AGEs) downregulate the miR-4429/PTEN axis to promote apoptosis of fibroblasts in pelvic organ prolapse.Ann Transl Med. 2022 Aug;10(15):821. doi: 10.21037/atm-22-628. Ann Transl Med. 2022. PMID: 36035012 Free PMC article.
-
Roles and mechanisms of biomechanical-biochemical coupling in pelvic organ prolapse.Front Med (Lausanne). 2024 Feb 12;11:1303044. doi: 10.3389/fmed.2024.1303044. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38410754 Free PMC article. Review.
-
Role of the integrin‑β1/TGF‑β1 signaling pathway in the pathogenesis of pelvic organ prolapse: A study on vaginal wall tissue alterations and molecular dysfunction.Mol Med Rep. 2025 Apr;31(4):104. doi: 10.3892/mmr.2025.13469. Epub 2025 Feb 21. Mol Med Rep. 2025. PMID: 39981910 Free PMC article.
-
An Improved Understanding of the Pathophysiology of Pelvic Organ Prolapse: A 3D In Vitro Model under Static and Mechanical Loading Conditions.Adv Healthc Mater. 2024 Mar;13(8):e2302905. doi: 10.1002/adhm.202302905. Epub 2024 Jan 26. Adv Healthc Mater. 2024. PMID: 38219051 Free PMC article.
-
The mechanism of adipose mesenchymal stem cells to stabilize the immune microenvironment of pelvic floor injury by regulating pyroptosis and promoting tissue repair.Mater Today Bio. 2023 Dec 18;24:100910. doi: 10.1016/j.mtbio.2023.100910. eCollection 2024 Feb. Mater Today Bio. 2023. PMID: 38204481 Free PMC article.
References
-
- Lang JH, Zhu L. Female Pelvisology. Beijing: People’s Medical Publishing House; 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

