Blood group O convalescent plasma donations have significantly lower levels of SARS-CoV-2 IgG antibodies compared to blood group A donations
- PMID: 34036595
- PMCID: PMC8242504
- DOI: 10.1111/trf.16524
Blood group O convalescent plasma donations have significantly lower levels of SARS-CoV-2 IgG antibodies compared to blood group A donations
Abstract
Background: COVID-19 convalescent plasma (CCP) is plasma collected from individuals who have recovered from SARS-CoV-2 infection. The FDA Emergency Use Authorization restricts use of CCP to high-titer units only. The purpose of this study was to determine if donor ABO blood group was associated with SARS-CoV-2 antibody response, and subsequent qualification as high-titer CCP.
Methods: All CCP donations collected from April 21, 2020 to September 1, 2020 were included. The Abbott ARCHITECT semi-quantitative chemiluminescent microparticle immunoassay was used to assess IgG antibodies to the nucleocapsid protein of SARS-CoV-2. Units with a S/C value ≥4.5 were considered high titer.
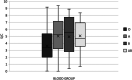
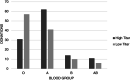
Results: A total of 232 CCP donations were evaluated. There were no significant differences in the distribution of sex, age, and interval from symptom resolution to donation by ABO blood group. The mean SARS-CoV-2 IgG antibody S/C value was significantly lower in blood group O donations (3.6), compared to blood group A (5.0) donations (p < .001). There was no difference in antibody response between the other blood group pairings. Blood group O donations resulted in a lower percentage of high-titer units (35%), compared to blood group A (60%), B (58%), and AB (65%) donations.
Conclusion: Blood group O donations were found to have significantly lower levels of SARS-CoV-2 IgG nucleocapsid antibodies compared to blood group A donations and were less likely to produce CCP units that qualified as high titer. These findings may aid donor recruitment to promote availability of high-titer CCP to meet patient needs.
Keywords: ABO blood group; Covid-19 convalescent plasma; SARS-CoV-2; antibody titer.
© 2021 AABB.
Conflict of interest statement
The authors have disclosed no conflicts of interest.
Figures
References
-
- Hinton D. Emergency use authorization for COVID‐19 convalescent plasma: letter of authorization [monograph on the internet]. U.S. Food and Drug Administration. 2021. https://www.fda.gov/media/141477/download. Accessed March 8, 2021.
-
- ARCHITECT SARS‐CoV‐2 IgG Package Insert [monograph on the internet]. Abbott Laboratories. 2020. https://www.fda.gov/media/137383/download. Accessed June 17, 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

