Hypothermic oxygenated machine perfusion of the human pancreas for clinical islet isolation: a prospective feasibility study
- PMID: 34036616
- PMCID: PMC8456912
- DOI: 10.1111/tri.13927
Hypothermic oxygenated machine perfusion of the human pancreas for clinical islet isolation: a prospective feasibility study
Abstract
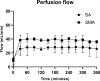
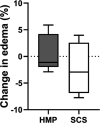
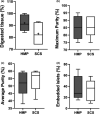
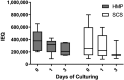
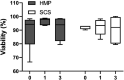
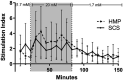
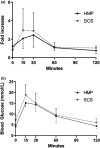
Due to an increasing scarcity of pancreases with optimal donor characteristics, islet isolation centers utilize pancreases from extended criteria donors, such as from donation after circulatory death (DCD) donors, which are particularly susceptible to prolonged cold ischemia time (CIT). We hypothesized that hypothermic machine perfusion (HMP) can safely increase CIT. Five human DCD pancreases were subjected to 6 h of oxygenated HMP. Perfusion parameters, apoptosis, and edema were measured prior to islet isolation. Five human DBD pancreases were evaluated after static cold storage (SCS). Islet viability, and in vitro and in vivo functionality in diabetic mice were analyzed. Islets were isolated from HMP pancreases after 13.4 h [12.9-14.5] CIT and after 9.2 h [6.5-12.5] CIT from SCS pancreases. Histological analysis of the pancreatic tissue showed that HMP did not induce edema nor apoptosis. Islets maintained >90% viable during culture, and an appropriate in vitro and in vivo function in mice was demonstrated after HMP. The current study design does not permit to demonstrate that oxygenated HMP allows for cold ischemia extension; however, the successful isolation of functional islets from discarded human DCD pancreases after performing 6 h of oxygenated HMP indicates that oxygenated HMP may be a useful technology for better preservation of pancreases.
Keywords: donation after circulatory death; hypothermic machine perfusion; islet isolation.
© 2021 The Authors. Transplant International published by John Wiley & Sons Ltd on behalf of Steunstichting ESOT.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017; 13: 268. - PubMed
-
- Nijhoff MF, Engelse MA, Dubbeld J, et al. Glycemic stability through islet‐after‐kidney transplantation using an alemtuzumab‐based induction regimen and long‐term triple‐maintenance immunosuppression. Am J Transplant 2016; 16: 246. - PubMed
-
- Eurotransplant chapter 7: ET pancreas allocation system (EPAS). Updated 2019.
-
- Proneth A, Schnitzbauer AA, Schenker P, et al. Extended pancreas donor program‐the EXPAND study: a prospective multicenter trial testing the use of pancreas donors older than 50 years. Transplantation 2018; 102: 1330. - PubMed
-
- Www.eurotransplant.org/statistics. Accessed May 6, 2020.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

