Bis(4-benzhydryl-benzoxazol-2-yl)methane - from a Bulky NacNac Alternative to a Trianion in Alkali Metal Complexes
- PMID: 34036637
- PMCID: PMC8361911
- DOI: 10.1002/chem.202100616
Bis(4-benzhydryl-benzoxazol-2-yl)methane - from a Bulky NacNac Alternative to a Trianion in Alkali Metal Complexes
Abstract
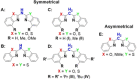
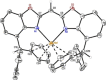
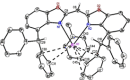
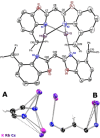
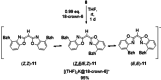
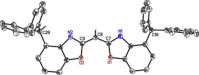
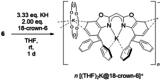
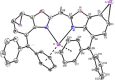
A novel sterically demanding bis(4-benzhydryl-benzoxazol-2-yl)methane ligand 6 (4-BzhH2 BoxCH2 ) was gained in a straightforward six-step synthesis. Starting from this ligand monomeric [M(4-BzhH2 BoxCH)] (M=Na (7), K (81 )) and dimeric [{M(4-BzhH2 BoxCH)}2 ] (M=K (82 ), Rb (9), Cs (10)) alkali metal complexes were synthesised by deprotonation. Abstraction of the potassium ion of 8 by reaction with 18-crown-6 resulted in the solvent separated ion pair [{(THF)2 K@(18-crown-6)}{bis(4-benzhydryl-benzoxazol-2-yl)methanide}] (11), including the energetically favoured monoanionic (E,E)-(4-BzhH2 BoxCH) ligand. Further reaction of 4-BzhH2 BoxCH2 with three equivalents KH and two equivalents 18-crown-6 yielded polymeric [{(THF)2 K@(18-crown-6)}{K@(18-crown-6)K(4-Bzh BoxCH)}]n (n→∞) (12) containing a trianionic ligand. The neutral ligand and herein reported alkali complexes were characterised by single X-ray analyses identifying the latter as a promising precursor for low-valent main group complexes.
Keywords: Ligand design; Metalation; Methanides; NacNac; Potassium; s-Block chemistry.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- Bourget-Merle L., Lappert M. F., Severn J. R., Chem. Rev. 2002, 102, 3031–3066; - PubMed
-
- Tsai Y.-C., Coord. Chem. Rev. 2012, 256, 722–758.
-
- F. T. Edelmann, Advances in the Coordination Chemistry of Amidinate and Guanidinate Ligands; Elsevier, Burlington, 2008, p. 184–339.
-
- Webster R. L., Dalton Trans. 2017, 46, 4483–4498. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

