Potentiation of glutamatergic synaptic transmission onto lateral habenula neurons following early life stress and intravenous morphine self-administration in rats
- PMID: 34036710
- PMCID: PMC8613295
- DOI: 10.1111/adb.13064
Potentiation of glutamatergic synaptic transmission onto lateral habenula neurons following early life stress and intravenous morphine self-administration in rats
Abstract
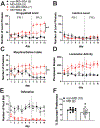
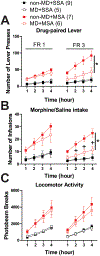
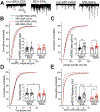
Early life stress presents an important risk factor for drug addiction and comorbid depression and anxiety through persistent effects on the mesolimbic dopamine pathways. Using an early life stress model for child neglect (a single 24 h episode of maternal deprivation, MD) in rats, recent published works from our lab show that MD induces dysfunction in the ventral tegmental area and its negative controller, the lateral habenula (LHb). MD-induced potentiation of glutamatergic synaptic transmission onto LHb neurons shifts the coordination of excitation/inhibition (E/I) balance towards excitation, resulting in an increase in the overall spontaneous neuronal activity with elevation in bursting and tonic firing, and in the intrinsic excitability of LHb neurons in early adolescent male rats. Here, we explored how MD affects intravenous morphine self-administration (MSA) acquisition and sucrose preference as well as glutamatergic synaptic function in LHb neurons of adult male rats self-administering morphine. We found that MD-induced increases in LHb neuronal and glutamatergic synaptic activity and E/I ratio persisted into adulthood. Moreover, MD significantly reduced morphine intake, triggered anhedonia-like behaviour in the sucrose preference test and was associated with persistent glutamatergic potentiation 24 h after the last MSA session. MSA also altered the decay time kinetics of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor (AMPAR) currents in LHb neurons of control rats during this time period. Our data highlight that early life stress-induced glutamatergic plasticity in LHb may dampen the positive reinforcing and motivational properties of both natural rewards and opioids, and may contribute to the development of anhedonia and dysphoric states associated with opioids.
Keywords: LHb; early life stress; glutamatergic synaptic transmission; lateral habenula; morphine self-administration.
Published 2021. This article is a U.S. Government work and is in the public domain in the USA.
Figures
Similar articles
-
Early life stress dysregulates kappa opioid receptor signaling within the lateral habenula.Neurobiol Stress. 2020 Nov 17;13:100267. doi: 10.1016/j.ynstr.2020.100267. eCollection 2020 Nov. Neurobiol Stress. 2020. PMID: 33344720 Free PMC article.
-
Dopamine D4 receptor excitation of lateral habenula neurons via multiple cellular mechanisms.J Neurosci. 2013 Oct 23;33(43):16853-64. doi: 10.1523/JNEUROSCI.1844-13.2013. J Neurosci. 2013. PMID: 24155292 Free PMC article.
-
Ethanol potentiates both GABAergic and glutamatergic signaling in the lateral habenula.Neuropharmacology. 2017 Feb;113(Pt A):178-187. doi: 10.1016/j.neuropharm.2016.09.026. Epub 2016 Sep 25. Neuropharmacology. 2017. PMID: 27678415
-
Synaptic and cellular profile of neurons in the lateral habenula.Front Hum Neurosci. 2013 Dec 16;7:860. doi: 10.3389/fnhum.2013.00860. Front Hum Neurosci. 2013. PMID: 24379770 Free PMC article. Review.
-
Ventral pallidal modulation of aversion processing.Brain Res. 2019 Jun 15;1713:62-69. doi: 10.1016/j.brainres.2018.10.010. Epub 2018 Oct 6. Brain Res. 2019. PMID: 30300634 Review.
Cited by
-
Effects of Repetitive Mild Traumatic Brain Injury on Corticotropin-Releasing Factor Modulation of Lateral Habenula Excitability and Motivated Behavior.bioRxiv [Preprint]. 2024 May 14:2024.04.16.589760. doi: 10.1101/2024.04.16.589760. bioRxiv. 2024. Update in: J Neurotrauma. 2025 May;42(9-10):832-850. doi: 10.1089/neu.2024.0184. PMID: 38798343 Free PMC article. Updated. Preprint.
-
Influence of Early-Life Stress on the Excitability of Dynorphin Neurons in the Adult Mouse Dorsal Horn.J Pain. 2024 Oct;25(10):104609. doi: 10.1016/j.jpain.2024.104609. Epub 2024 Jun 15. J Pain. 2024. PMID: 38885917
-
Tachykinin receptor 3 in the lateral habenula alleviates pain and anxiety comorbidity in mice.Front Immunol. 2023 Jan 23;14:1049739. doi: 10.3389/fimmu.2023.1049739. eCollection 2023. Front Immunol. 2023. PMID: 36756128 Free PMC article.
-
Stress-induced anxiety-related behavior in mice is driven by enhanced excitability of ventral tegmental area GABA neurons.Front Behav Neurosci. 2024 Jul 17;18:1425607. doi: 10.3389/fnbeh.2024.1425607. eCollection 2024. Front Behav Neurosci. 2024. PMID: 39086371 Free PMC article.
-
Involvement of Lateral Habenula Dysfunction in Repetitive Mild Traumatic Brain Injury-Induced Motivational Deficits.J Neurotrauma. 2023 Jan;40(1-2):125-140. doi: 10.1089/neu.2022.0224. Epub 2022 Sep 22. J Neurotrauma. 2023. PMID: 35972745 Free PMC article.
References
-
- Authement ME, Kodangattil JN, Gouty S, et al. Histone Deacetylase Inhibition Rescues Maternal Deprivation-Induced GABAergic Metaplasticity through Restoration of AKAP Signaling. Neuron. 2015;86(5):1240–1252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

