DDRugging glioblastoma: understanding and targeting the DNA damage response to improve future therapies
- PMID: 34036721
- PMCID: PMC8732357
- DOI: 10.1002/1878-0261.13020
DDRugging glioblastoma: understanding and targeting the DNA damage response to improve future therapies
Abstract
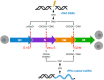
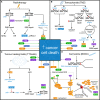
Glioblastoma is the most frequently diagnosed type of primary brain tumour in adults. These aggressive tumours are characterised by inherent treatment resistance and disease progression, contributing to ~ 190 000 brain tumour-related deaths globally each year. Current therapeutic interventions consist of surgical resection followed by radiotherapy and temozolomide chemotherapy, but average survival is typically around 1 year, with < 10% of patients surviving more than 5 years. Recently, a fourth treatment modality of intermediate-frequency low-intensity electric fields [called tumour-treating fields (TTFields)] was clinically approved for glioblastoma in some countries after it was found to increase median overall survival rates by ~ 5 months in a phase III randomised clinical trial. However, beyond these treatments, attempts to establish more effective therapies have yielded little improvement in survival for patients over the last 50 years. This is in contrast to many other types of cancer and highlights glioblastoma as a recognised tumour of unmet clinical need. Previous work has revealed that glioblastomas contain stem cell-like subpopulations that exhibit heightened expression of DNA damage response (DDR) factors, contributing to therapy resistance and disease relapse. Given that radiotherapy, chemotherapy and TTFields-based therapies all impact DDR mechanisms, this Review will focus on our current knowledge of the role of the DDR in glioblastoma biology and treatment. We also discuss the potential of effective multimodal targeting of the DDR combined with standard-of-care therapies, as well as emerging therapeutic targets, in providing much-needed improvements in survival rates for patients.
Keywords: DNA damage response; chemotherapy; glioblastoma; radiotherapy; synthetic lethality; tumour-treating fields.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
OR and SJC have received research funding from the funding bodies acknowledged below and are recipients of an Inovitro™ TTFields preclinical research system (on loan from Novocure) and take part in the annual Inovitro™ Users Meeting hosted by Novocure.
Figures

References
-
- Patel AP, Fisher JL, Nichols E, Abd‐Allah F, Abdela J, Abdelalim A, Abraha HN, Agius D, Alahdab F, Alam T et al. (2019) Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 376–393. - PMC - PubMed
-
- Reifenberger G, Wirsching HG, Knobbe‐Thomsen CB & Weller M (2017) Advances in the molecular genetics of gliomas – implications for classification and therapy. Nat Rev Clin Oncol 14, 434–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

