Phenotypic plasticity underlies local invasion and distant metastasis in colon cancer
- PMID: 34036938
- PMCID: PMC8192123
- DOI: 10.7554/eLife.61461
Phenotypic plasticity underlies local invasion and distant metastasis in colon cancer
Abstract
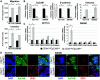
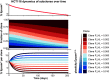
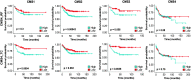
Phenotypic plasticity represents the most relevant hallmark of the carcinoma cell as it bestows it with the capacity of transiently altering its morphological and functional features while en route to the metastatic site. However, the study of phenotypic plasticity is hindered by the rarity of these events within primary lesions and by the lack of experimental models. Here, we identified a subpopulation of phenotypic plastic colon cancer cells: EpCAMlo cells are motile, invasive, chemo-resistant, and highly metastatic. EpCAMlo bulk and single-cell RNAseq analysis indicated (1) enhanced Wnt/β-catenin signaling, (2) a broad spectrum of degrees of epithelial to mesenchymal transition (EMT) activation including hybrid E/M states (partial EMT) with highly plastic features, and (3) high correlation with the CMS4 subtype, accounting for colon cancer cases with poor prognosis and a pronounced stromal component. Of note, a signature of genes specifically expressed in EpCAMlo cancer cells is highly predictive of overall survival in tumors other than CMS4, thus highlighting the relevance of quasi-mesenchymal tumor cells across the spectrum of colon cancers. Enhanced Wnt and the downstream EMT activation represent key events in eliciting phenotypic plasticity along the invasive front of primary colon carcinomas. Distinct sets of epithelial and mesenchymal genes define transcriptional trajectories through which state transitions arise. pEMT cells, often earmarked by the extracellular matrix glycoprotein SPARC together with nuclear ZEB1 and β-catenin along the invasive front of primary colon carcinomas, are predicted to represent the origin of these (de)differentiation routes through biologically distinct cellular states and to underlie the phenotypic plasticity of colon cancer cells.
Keywords: cancer biology; colon cancer; human; partial EMT; phenotypic plasticity.
© 2021, Sacchetti et al.
Conflict of interest statement
AS, MW The authors of this manuscript do not have any competing financial interests in relation to the work described. MT, OS No competing interests declared
Figures















References
-
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. - DOI
-
- Biddle A, Liang X, Gammon L, Fazil B, Harper LJ, Emich H, Costea DE, Mackenzie IC. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Research. 2011;71:5317–5326. doi: 10.1158/0008-5472.CAN-11-1059. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

